Preparation method of terbutaline sulfate and B crystal form thereof
A technology of terbutaline sulfate and crystal form, which is applied in the field of chemical drug synthesis, can solve the problems of easy oxidation and deterioration of hydroxyl groups, fewer reaction steps, and high cost, and achieve industrial production, mild reaction conditions, and short reaction routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] In the present embodiment, the preparation method of terbutaline sulfate and its medicinal B crystal form is as follows:
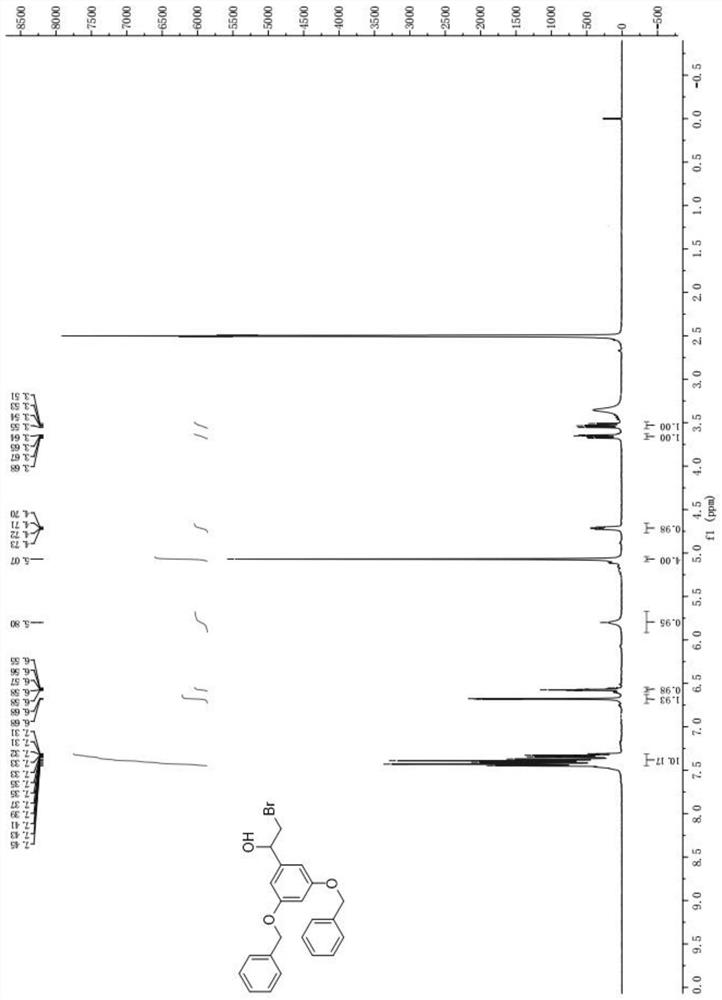
[0041] (1) Preparation of 1-[3,5-bis(benzyloxy)phenyl]-2-bromoethanol
[0042]At room temperature, add 100.0g (300.84mmol) of 3,5-dibenzyloxyacetophenone and 800mL of dichloromethane into the reaction vessel, stir to dissolve, add 141.1g (631.77mmol) of copper bromide, and stir under reflux for 3h. The reaction solution was suction filtered, the filtrate was washed with concentrated hydrochloric acid, and the organic layer was evaporated under reduced pressure to remove dichloromethane. Add 500mL methanol to the residue, stir and cool down to 10-15°C, add 16.3g (300.84mmol) potassium borohydride under temperature control, after the addition is complete, keep stirring for 1h. After adding 100mL of water, continue to stir for 30min, filter with suction, and wash the filter cake with water to obtain 121.8g of off-white solid, namely 1-[3,5-bis(benzylo...
Embodiment 2
[0055] The preparation method of present embodiment terbutaline sulfate and pharmaceutical B crystal form thereof is:
[0056] (1) Preparation of 1-[3,5-bis(benzyloxy)phenyl]-2-bromoethanol
[0057] At room temperature, add 100.0g (300.84mmol) of 3,5-dibenzyloxyacetophenone and 1L of chloroform into the reaction vessel, stir and dissolve, then add 72.1g (451.26mmol) of bromine dropwise at 20-25°C, After dropping, the temperature was raised to reflux and stirred for 1 h. The reaction solution was evaporated under reduced pressure to remove chloroform. Add 500mL methanol to the residue, stir and cool down to 10-15°C, add 17.1g (451.26mmol) sodium borohydride under temperature control, after the addition is complete, keep stirring for 1h. After adding 100mL of water, continue to stir for 30min, filter with suction, and wash the filter cake with water to obtain 109.4g of off-white solid, that is, 1-[3,5-bis(benzyloxy)phenyl]-2-bromoethanol, yield 88 %.
[0058] (2) Preparation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com