Stable terbutaline sulfate injection and preparation process thereof
A technology of terbutaline and injection, which is applied in the fields of drug delivery, respiratory system diseases, inorganic non-active ingredients, etc., can solve the problems of hidden safety hazards, excessive time, toxic and side effects of clinical application of injection, and reduce metal ions. Complexing agents, reducing the use of antioxidants, and ensuring the effect of clinically safe medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Prescription: Terbutaline Sulfate 0.25g
[0028] Add water for injection to 1000ml.
[0029] Preparation process: brief description: water for injection with prescription quantity 98, temperature 40°C, add sodium chloride and terbutaline sulfate at one time, stir to dissolve, add activated carbon with 0.3% sodium chloride, stir for 15 minutes, decarburize, filter , the filtrate was adjusted to pH 4.0. It was filtered through a 0.22um pore-size terminal filter, filled in neutral borosilicate glass ampoules, each filled with 1ml, and sterilized by moist heat at 115°C for 35 minutes to obtain the product.
Embodiment 2
[0031] Prescription: Terbutaline Sulfate 0.20g
[0032] Sodium chloride 8.5g
[0033] Add water for injection to 1000ml.
[0034] The preparation process is the same as in Example 1.
Embodiment 1-2
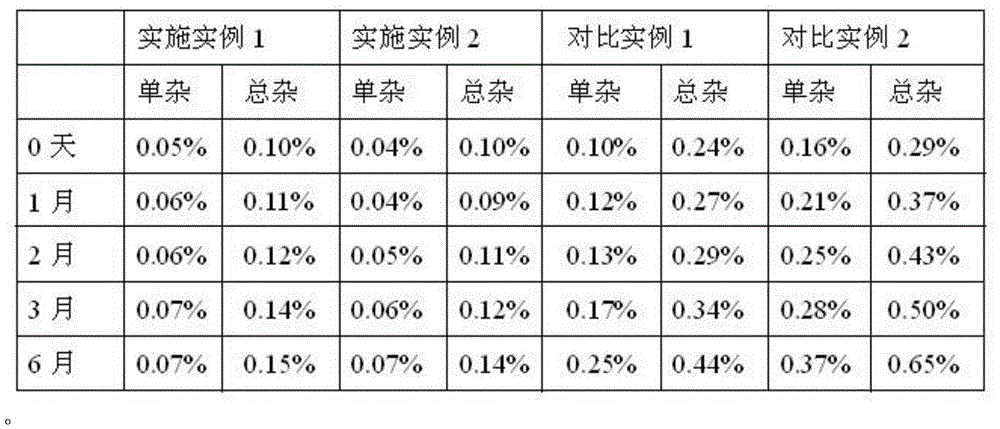
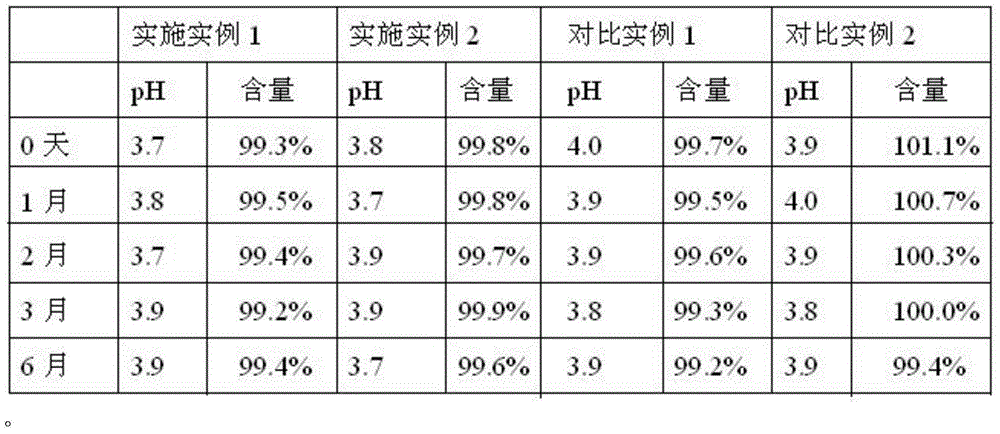
[0036] Example 1-2 Stability investigation setting comparative example 1-2:
[0037] Prescription: Terbutaline Sulfate 0.25g
[0038] Sodium metabisulfite 0.25g
[0039] EDTA-2Na 0.1g
[0040] Sodium chloride 8.5g
[0041] Add water for injection to 1000ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com