Preparation method of optically-active 7-halogenated-6-hydroxyheptane-3-ethylene-2-ketone
An optically active, hydroxyheptane technology, which is applied in the field of preparation of optically active 7-halo-6-hydroxyhept-3-en-2-one, can solve the problems of harsh reaction conditions, high price, and high cost, and achieve the goal of preparing Simple, Wide-Source Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
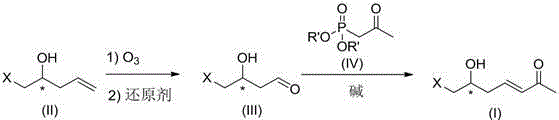
[0027] Will( S )-5-Chloro-4-hydroxy-1-pentene (1.2 g) was dissolved in dichloromethane (25 ml), cooled to -40 °C with stirring, and ozone was introduced until the reaction solution turned blue ( for about 10 min), air was introduced until the blue color disappeared, a saturated aqueous solution of sodium thiosulfate (10 mL) was added, it was naturally raised to room temperature under stirring, extracted with dichloromethane, the organic layers were combined, dried over anhydrous sodium sulfate, and reduced Concentrate under pressure to get ( S )-4-Chloro-3-hydroxybutyraldehyde (1.13 g, 93%). The product was directly used in the next step reaction.
Embodiment 2
[0029] Will( R )-5-Chloro-4-hydroxy-1-pentene (1.2 g) was dissolved in dichloromethane (25 ml), cooled to -80 °C with stirring, and ozone was introduced until the reaction solution turned blue ( for about 10 min), air was introduced until the blue color disappeared, dimethyl sulfide (5 mL) was added, and it was naturally raised to room temperature under stirring, washed with saturated brine, the organic layer was dried over anhydrous sodium sulfate, and concentrated under reduced pressure. have to( R )-4-Chloro-3-hydroxybutyraldehyde (1.17 g, 96%). The product was directly used in the next step reaction.
Embodiment 3
[0031] Potassium tert-butoxide (0.6 g) was dissolved in tetrahydrofuran (20 mL), and 2-oxopropyl diethyl phosphate (1.0 g) was added dropwise with stirring at 0°C. add( S )-4-Chloro-3-hydroxybutyraldehyde (0.61 g) dissolved in tetrahydrofuran (5 mL) solution, after dropping, stir at room temperature for 10 h, after the reaction is complete, pour the reaction solution into saturated ammonium chloride solution, and use extracted with ethyl acetate, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain ( S )-7-Chloro-6-hydroxy-hept-3-en-2-one (0.80 g, 99%), =2.0 o ( c 0.94, CHCl 3 )
[0032] 1 H NMR (400MHz, CDCl 3 ): δ 6.79 (dt, 1H), 6.12 (d, 1H), 3.94 (m, 1H), 3.54 (dd, 1H), 3.48 (dd, 1H), 3.17 (br, 1H), 2.46 (m, 2H ), 2.21 (s, 3H).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap