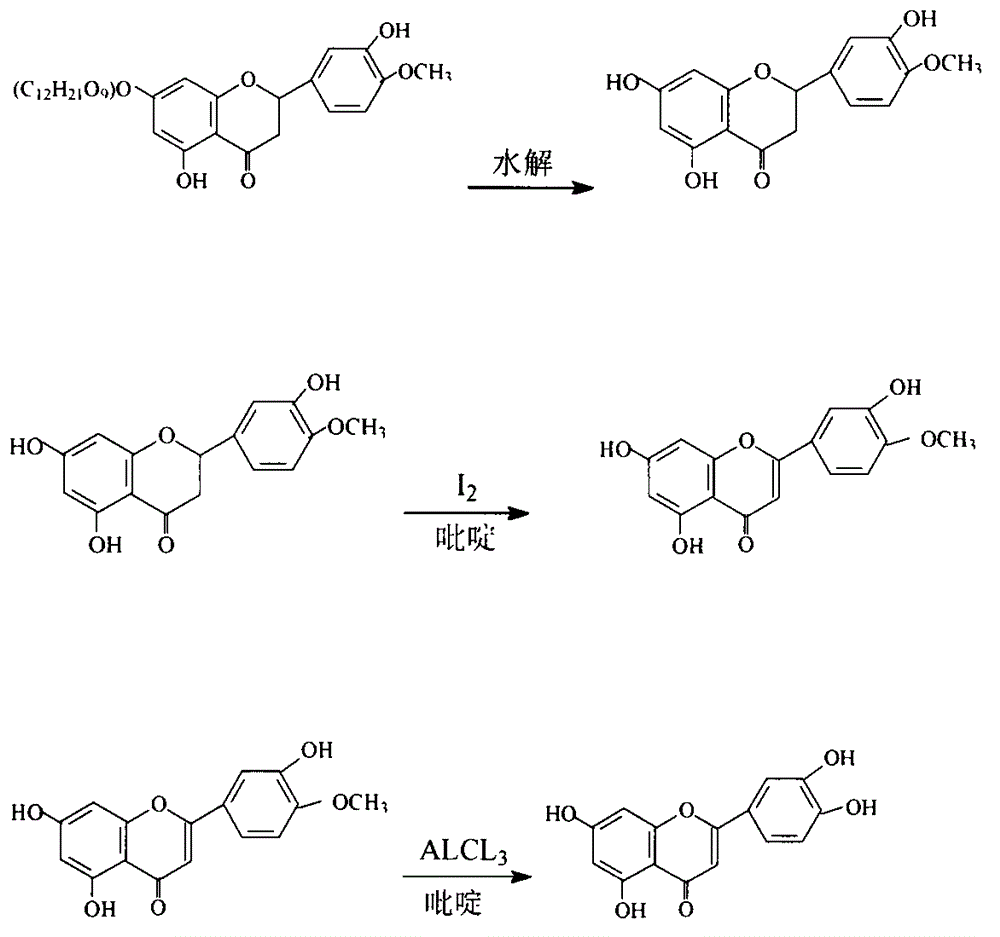
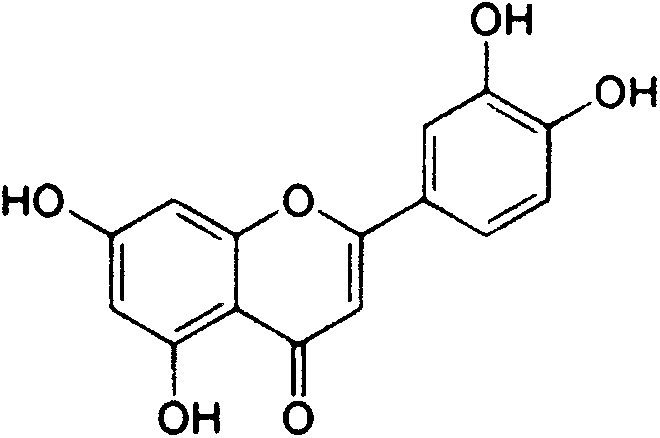
New technology for preparing luteolin by using hesperidin
A technology of luteolin and hesperidin, applied in the direction of organic chemistry, etc., can solve the problems of cumbersome process, low yield and low output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] Preparation of the first hesperidin: close the mouth of the reaction kettle, pump 1500-1700Kg of ethanol with accurate measurement into the reaction kettle, the ethanol has been adjusted to pH 1-2 with concentrated sulfuric acid, slowly add 120Kg of crude hesperidin from the reaction kettle mouth, and seal the reaction At the kettle mouth, start stirring, open the heat-carrying steam valve of the reaction kettle to heat, open the circulating condensed water, and reflux at 70-75°C for reaction. After reacting for 12 hours, take a sample from the sampling port for detection. After the reaction is complete, start to concentrate until the purity of the concentrated solution reaches 15-25%. ℃ dry 4 ~ 8h. Obtain the crude product of hesperetin. Add 900Kg of 95% ethanol to the reaction kettle, put 100Kg of crude hesperetin into it, then add 3kg of activated carbon, close the mouth of the kettle, open the heat carrying steam valve of the reaction kettle, and wait until the tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com