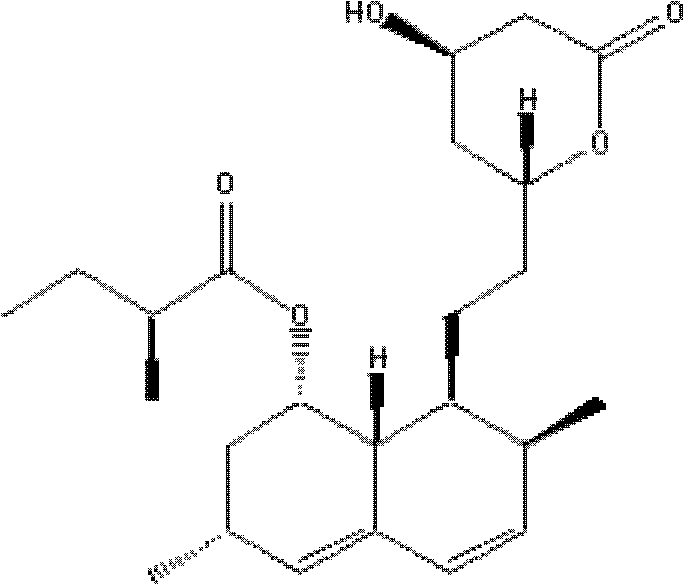
Lovastatin purification method combining auxiliary magnetic crystallization
A purification method, lovastatin technology, applied in the field of further separation and purification of lovastatin crude product through crystallization, which can solve the problem of low product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 80g crude lovastatin was thermally dissolved in 250ml ethanol, 1.4g activated carbon was added, hot filtered, the volume was set to 280ml, divided into two parts, one part was left to stand for crystallization; Gradually cool until a large number of crystals are precipitated, stop the magnetic assistance, continue to cool to 10 ° C, suction filtration, and vacuum dry to obtain the finished product.
Embodiment 2
[0021] 80g crude lovastatin was thermally dissolved in 200ml ethanol, 1.4g activated carbon was added, hot filtered, the volume was set to 230ml, divided into two parts, one part was left to stand for crystallization; Gradually cool until a large number of crystals are precipitated, stop the magnetic assistance, continue to cool to 15 ° C, suction filtration, and vacuum dry to obtain the finished product.
Embodiment 3
[0023] 80g crude lovastatin was thermally dissolved in 300ml ethanol, 1.4g activated carbon was added, hot filtered, the volume was set to 320ml, divided into two parts, one part was left to crystallize; Gradually cool until a large number of crystals are precipitated, stop the magnetic assistance, continue to cool to 5°C, suction filtration, and vacuum dry to obtain the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com