Organosiloxane containing acid anhydride group and method for preparing the same
An organosiloxane and acid anhydride-based technology, applied in the field of organosiloxane, can solve the problems of reduced purity, low hydrophilicity, and difficulty in the affinity of inorganic substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
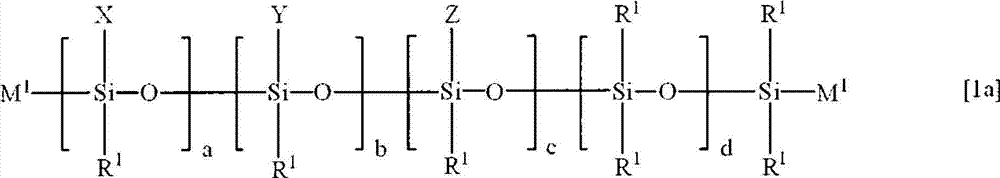
[0118] In a 1-liter 3-necked flask equipped with a stirrer, a thermometer, and a Jimrot cooling tube, 100 g (0.192 mol) of an organohydrogensiloxane represented by the following formula [18] and 114 g of toluene were charged, and chloroplatinum was added while stirring. 1.00 g of the toluene solution (Pt concentration: 0.5% by mass) of the acid. Next, the temperature was raised to 80° C., and 56.7 g (0.383 mol) of vinyltrimethoxysilane was added dropwise, followed by aging for 2 hours.
[0119]
[0120] Here, the reaction rate of the vinyltrimethoxysilane used for the said reaction was measured as follows. First, the ≡SiH content in 1 g of the sample before and after the reaction was measured by the following method. To 1 g of the samples before and after the reaction, 10 g of butanol was respectively added, and further, 20 g of a 20% by mass NaOH aqueous solution was added while stirring. The hydrogen gas generated at this time (≡SiH+H 2 O→≡SiOH+H 2 ↑) to calculate the...
Embodiment 2
[0153] In the same manner as in Example 1, after charging 100 g (0.192 mol) of organohydrogensiloxane represented by the above formula [18] and 114 g of toluene, a toluene solution of chloroplatinic acid (Pt concentration: 0.5% by mass) was added while stirring. 1.00g. Next, the temperature was raised to 80° C., and 56.7 g (0.383 mol) of vinyltrimethoxysilane was added dropwise, followed by aging for 2 hours.
[0154] Next, an operation for reacting the allyl polyether with a part of the remaining ≡SiH groups in the methylhydrogensiloxane was performed. With the reaction liquid maintained at 80° C., 23.0 g (0.0962 mol) of allyl polyether represented by the following formula was added dropwise with stirring, and aging was performed for further 3 hours.
[0155] CH 2 =CH-CH 2 -O(CH 2 CH 2 O) 3.8 CH 3
[0156] Here, the reaction rate of allyl polyether was measured. In the same manner as in Example 1, the ≡SiH content in 1 g of the reaction liquid before and after the re...
Embodiment 3
[0186] In Example 2, except CH 2 =CH-CH 2 -O(CH 2 CH 2 O) 3.8 CH 3 The addition amount of the indicated compound was changed from 23.0 g (0.0962 mol) to 46.0 g (0.192 mol), and the addition amount of allyl succinic anhydride was changed from 120 g (0.857 mol) to 90.5 g (0.646 mol). performed the same operation.
[0187] First, the reaction rate of allyl polyether was measured. In the same manner as in Examples 1 and 2, the ≡SiH content in 1 g of the reaction liquid before and after the reaction was measured, and the amount of allyl polyether actually reacted was calculated. The results are shown in Table 5.
[0188] [table 5]
[0189] Reaction amount of allyl polyether
[0190]
[0191] In 1g sample before reaction, there is 0.605×10 -3 mol of allyl polyether charged as raw material. The reaction rate of the allyl polyether was calculated as follows from the reaction amount obtained above and the amount charged as the raw material, and it was 95.9%.
[0192] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com