Organosiloxane containing anhydride groups and manufacturing method thereof
An organosiloxane, a technology for a manufacturing method, applied in organic chemistry, organic silicon compounds, chemical instruments and methods, etc., can solve problems such as low hydrophilicity, reduced purity, and changes over time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Example 1 (Manufacture of succinic anhydride-modified methoxysiloxane)
[0180] Production of succinic anhydride-modified methoxysiloxane was carried out through the following two steps (A) and (B).
[0181] (A) Manufacturing process of methoxy group-containing methylhydrogen siloxane
[0182] Si 4 o 3 (OCH 3 ) 10 (In formula [1] stipulated in the present invention, R 2 =CH 3 , the partial hydrolysis condensate of alkoxysilane with n=0) after 85.0g (0.181mol) of methoxysiloxane and 43.4g (0.181mol) of tetramethyltetrahydrocyclotetrasiloxane, the side While stirring, 0.0646 g of trifluoromethanesulfonic acid was added, and the mixture was reacted at room temperature for 4 hours. After the reaction, add Mg to the system 6 Al 2 (OH) 16 CO 3 4H 2 0.388 g of a solid alkaline neutralizer represented by O was stirred for 2 hours, neutralized with trifluoromethanesulfonic acid, and purified by filtration to obtain 122 g of product-1 (methyl hydrogen silicon contai...
Embodiment 2
[0207] Example 2 (Manufacture of succinic anhydride / polyether co-modified methoxysiloxane-1)
[0208] Production of succinic anhydride / polyether co-modified methoxysiloxane was carried out through the step (A) of Example 1 and the following two steps of (C).
[0209] (C) Step of introducing polyether group and acid anhydride group into methoxy group-containing methylhydrogen siloxane
[0210] First, an operation for reacting an allyl polyether with a part of the ≡SiH group contained in the methoxy group-containing methylhydrogensiloxane is performed.
[0211] Into a 1-liter 3-neck flask equipped with a stirrer, a thermometer, and a Jimrot cooling tube, 120 g of the product-1 (methoxy-containing methylhydrogensiloxane) obtained in the step (A) of Example 1 and After 36 g of toluene, 1.50 g of a toluene solution (Pt concentration: 0.5% by mass) of chloroplatinic acid was added with stirring. Next, after raising the temperature to 90°C, the following formula
[0212] CH 2 =...
Embodiment 3
[0240] Example 3 (Manufacture of succinic anhydride / polyether co-modified methoxysiloxane-2)
[0241] In Example 2, except CH 2 =CH-CH 2 -O(CH 2 CH 2 O) 3.8 CH 3 The same operation was performed except that the addition amount of the compound shown was changed from 20.3 g (0.0849 mol) to 40.6 g (0.170 mol).
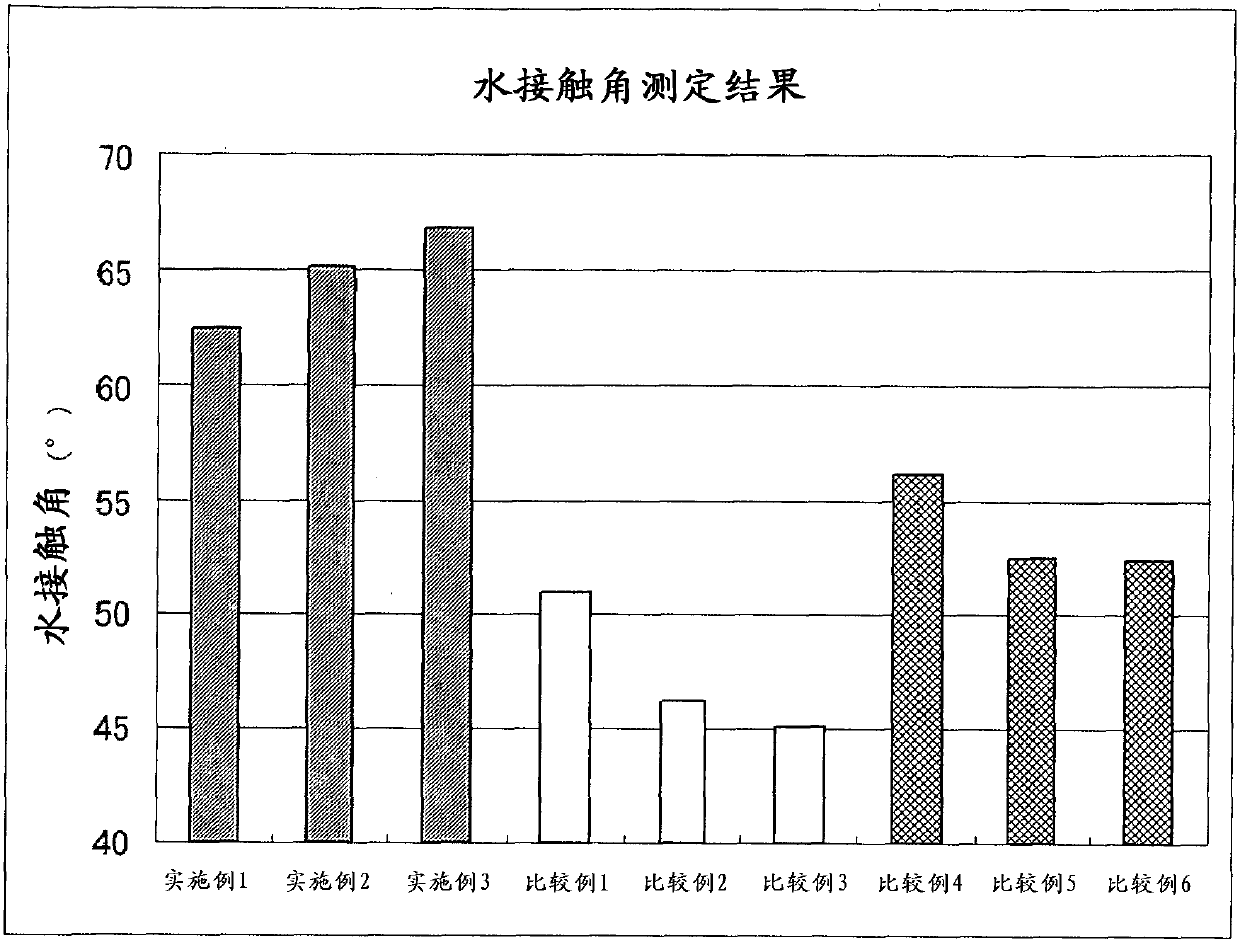
[0242] First, the reaction rate of the allyl polyether was measured after reacting the allyl polyether with a part of the ≡SiH groups contained in the methoxy group-containing methylhydrogensiloxane. In the same manner as in Examples 1 and 2, the ≡SiH content in 1 g of the reaction liquid before and after the reaction was measured, and the amount of allyl polyether actually reacted was calculated. The results are shown in Table 4.
[0243] [Table 4]
[0244] Reaction amount of allyl polyether
[0245]
[0246]In 1g sample before reaction, there is 0.857×10 -3 mol of allyl polyether charged as raw material. The reaction rate of the allyl polyether was calcu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com