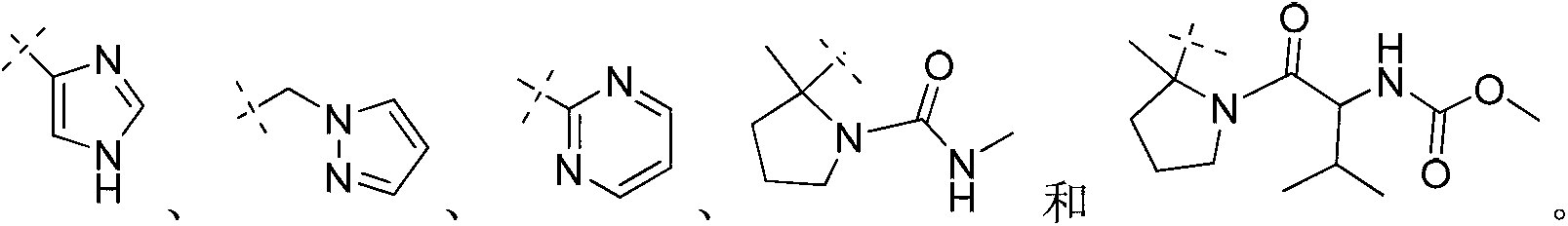
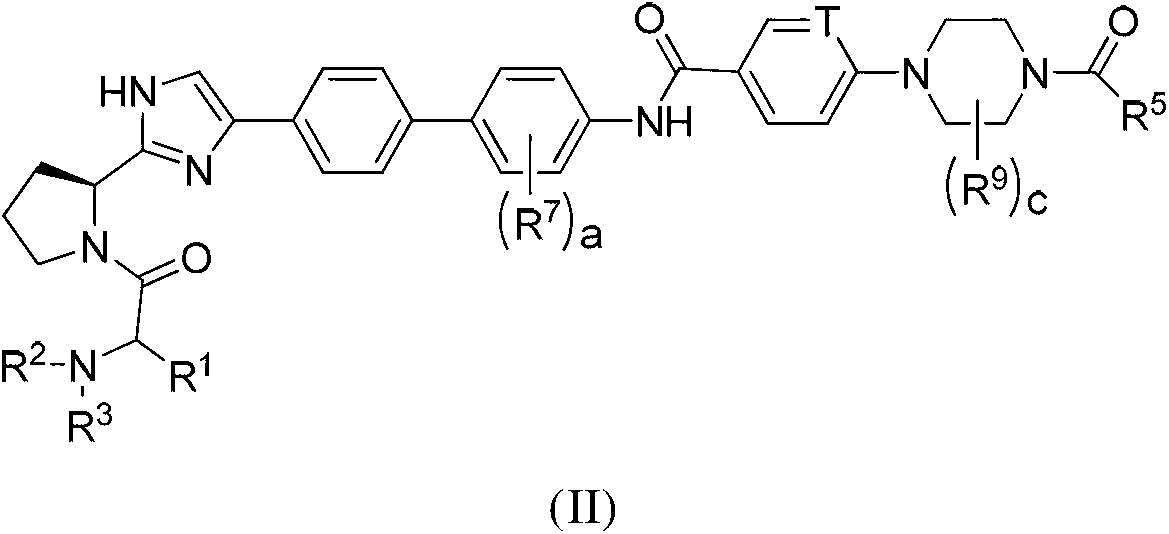
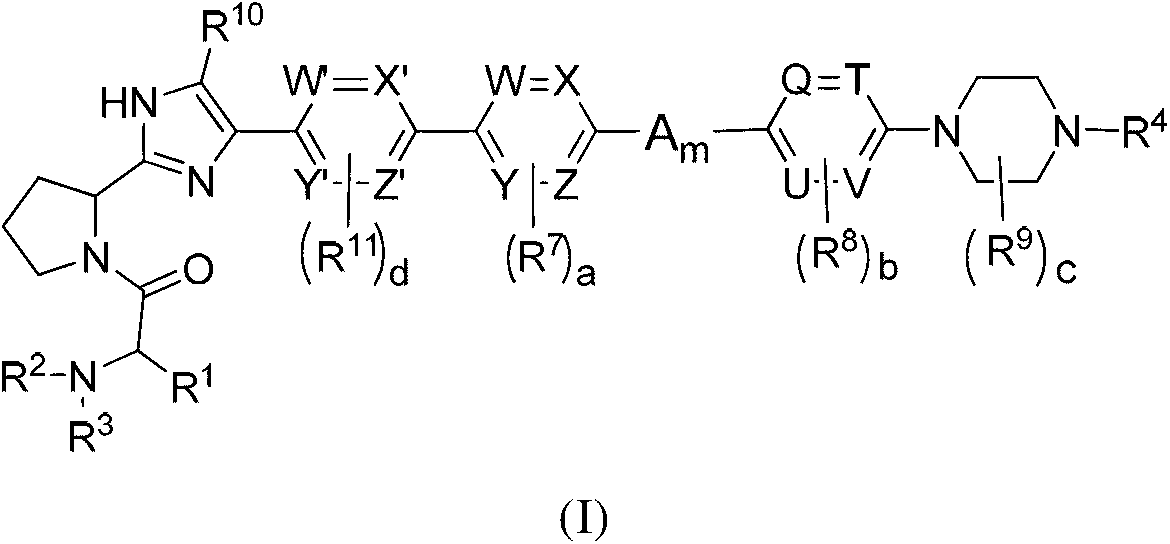Novel inhibitors of hepatitis C virus
A technology with limited conditions and alkyl groups, applied in the field of inhibitor compounds, can solve problems such as reported side effects, long treatment process, and unmet urgent needs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0307] The following synthetic examples and biological examples are provided to illustrate the invention and are not to be construed as limiting the scope of the invention in any way. In the following examples, the following abbreviations have the following meanings unless otherwise indicated. Abbreviations not defined below have their accepted meanings.
[0308] ACN = acetonitrile
[0309] DCM = dichloromethane
[0310] DMA = N, N-dimethylacetamide
[0311] DMF = N,N-Dimethylformamide
[0312] DMSO = dimethyl sulfoxide
[0313] EDC = N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride
[0314] EtOAc = ethyl acetate
[0315] h = hour
[0316] HATU = N, N, N', N'-tetramethyl-O-(7-azabenzotriazol-1-yl)uronium salt of hexafluorophosphate
[0317] HCTU = 2-(6-chloro-1H-benzotriazol-1-yl)-1,1,3,3-tetramethylammonium hexafluorophosphate
[0318] HOAt = 1-hydroxy-7-azabenzotriazole
[0319] min = minute
[0320] Pd(dppf)Cl 2 = Dichloro(1,1'-bis(diphenylphosph...
example 1
[0669] Example 1: {(S)-1-[(S)-2-(4-{4'-[4-(4-cyclopropanecarbonyl-piperazin-1-yl)-benzoylamino]-biphenyl -4-yl}-1H-imidazol-2-yl)-pyrrolidine-1-carbonyl]-2-methyl-propyl}-methyl carbamate
[0670]
[0671] To a solution of 4-(4-cyclopropanecarbonyl-piperazin-1-yl)-benzoic acid (0.015 g, 0.055 mmol) in dichloromethane (2 mL, 30 mmol) was added N,N-dimethylformamide (0.004 mL, 0.055 mmol) and oxalyl chloride (0.014 mL, 0.164 mmol). The reaction mixture was stirred for 25 minutes, followed by the sequential addition of N,N-diisopropylethylamine (0.048 mL, 0.273 mmol) and ((S)-1-{(S)-2-[4-(4'-amino -biphenyl-4-yl)-1H-imidazol-2-yl]-pyrrolidine-1-carbonyl}-2-methyl-propyl)-methyl carbamate (0.025 g, 0.055 mmol). The reaction mixture was stirred for 90 minutes, dried by rotary evaporation, dissolved in 1:1 acetic acid:water (1.5 mL) and purified by preparative HPLC to afford the trifluoroacetate salt of the title compound (7.9 mg). (m / z): C 41 h 47 N 7 o 5 [M+H] of + The ...
example 2
[0672] Example 2: ((S)-1-{(S)-2-[4-(4'-{4-[4-(2,2-Dimethyl-propionyl)-piperazin-1-yl] -Benzoylamino}-biphenyl-4-yl)-1H-imidazol-2-yl]-pyrrolidine-1-carbonyl}-2-methyl-propyl)-methyl carbamate
[0673]
[0674] Stir 4-[4-(2,2-Dimethyl-propionyl)-piperazin-1-yl]-benzoic acid (10.3 mg, 0.036 mmol), N-(3-dimethylaminopropyl)- N'-Ethylcarbodiimide hydrochloride (8.17mg, 0.043mmol), 1-hydroxy-7-azabenzotriazole (6.77mg, 0.050mmol) in dichloromethane (0.3mL, 4mmol) The reaction mixture was dissolved and stirred for another 20 minutes. Then add ((S)-1-{(S)-2-[4-(4′-amino-biphenyl-4-yl)-1H-imidazol-2-yl]-pyrrolidin-1- Carbonyl}-2-methyl-propyl)-methyl carbamate (8.2 mg, 0.018 mmol) and N,N-diisopropylethylamine (7.43 μL, 0.0426 mmol). The reaction mixture was stirred overnight, concentrated, dissolved in 1:1 acetic acid:water (1.5 mL) and purified by preparative HPLC to afford the title compound as the trifluoroacetate salt (5.6 mg). (m / z): C 42 h 51 N 7 o 5 [M+H] of + The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com