Method for synthetizing chiral cyclic alkyl amino acid by amino transferase
An aminotransferase and alkyl amino acid technology, which is applied in the field of synthesizing chiral cyclic alkyl amino acids by aminotransferase, can solve the problems of high production cost, high price, low yield and the like, and achieves mild reaction conditions and stable process. , Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
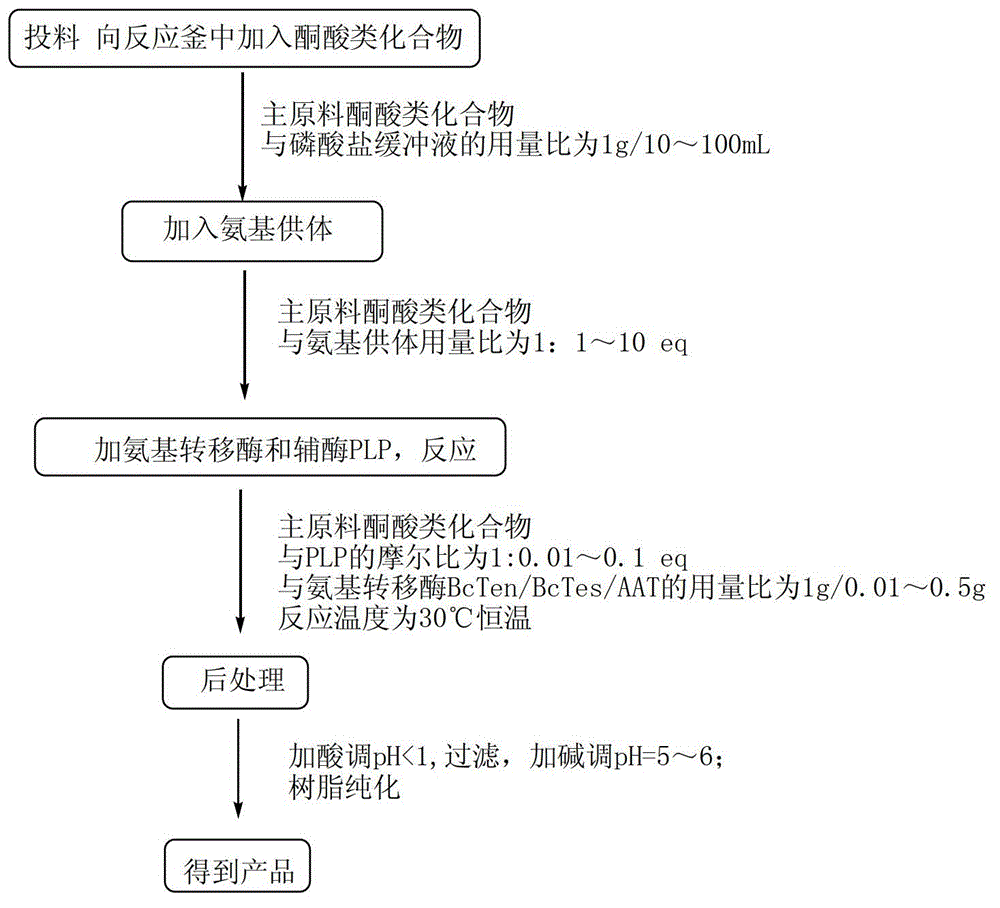
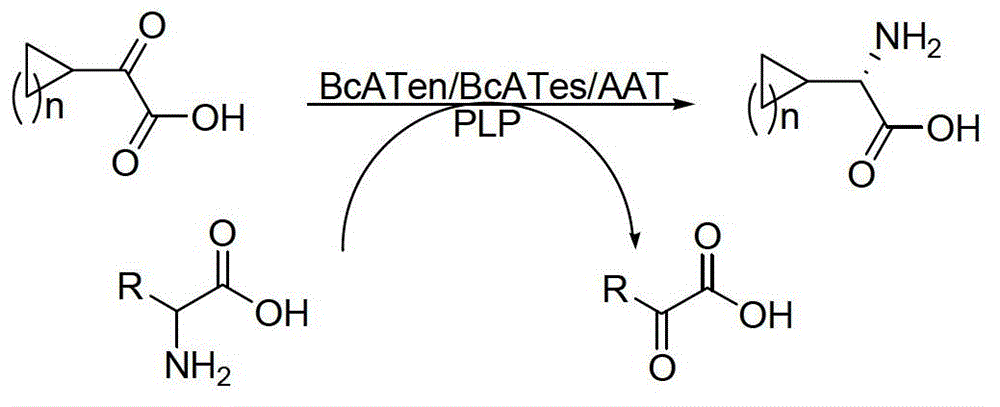
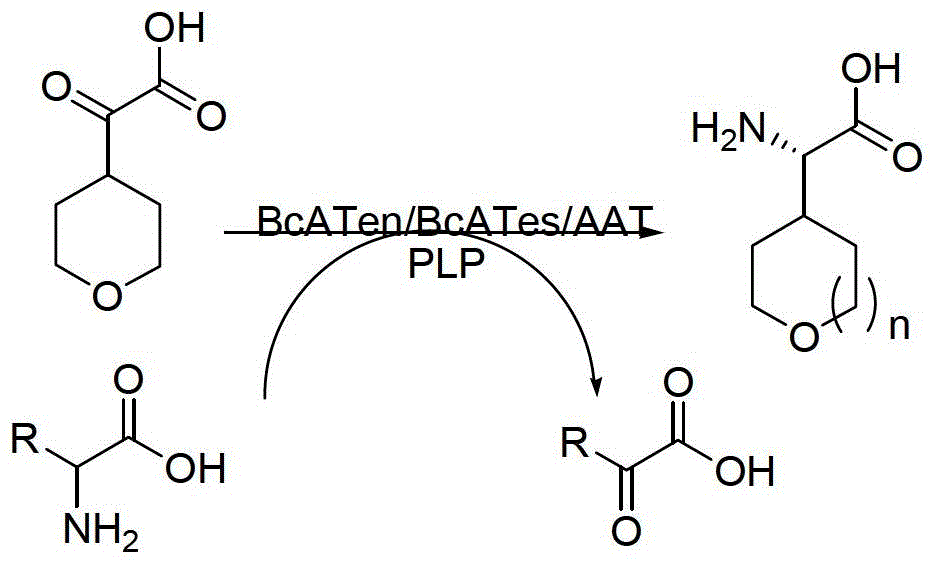
[0027] Embodiment 1: the method for utilizing aminotransferase to synthesize chiral cyclic alkyl amino acids is characterized in that the specific steps are as follows:
[0028] (1) Feeding: Add 2.12kg of the main raw material 2-cyclohexyl-2-oxoacetic acid sodium salt to a 200L reactor, add 100L of phosphate buffer (50mM, pH=7.0), and make the main raw material 2-cyclohexyl- 2-Oxyacetic acid sodium salt is uniformly dissolved in phosphate buffer;
[0029] (2) Add amino donor: Add 1.75kg of L-glutamic acid as amino donor to the 200L reactor, stir until completely dissolved, and adjust the system to pH=7.0;
[0030] (3) Add aminotransferase and coenzyme: Add 0.03kg coenzyme pyridoxal phosphate PLP and 0.21kg aminotransferase main enzyme BcATes to the 200L reactor;
[0031] (4) Reaction: The system was stirred in a reaction kettle at a stirring speed of 150 rpm, and kept at 30°C for 72 hours; the filtrate was concentrated, filtered with suction, and rinsed to obtain a solid crud...
Embodiment 2
[0033] Embodiment 2: the method for utilizing aminotransferase to synthesize chiral cyclic alkyl amino acid is characterized in that the specific steps are as follows:
[0034] (1) Feeding: Add 1.25kg of main raw material sodium cyclopentylpyruvate to a 200L reactor, and add 100L of phosphate buffer (100mM, pH=8.0), so that the main raw material sodium cyclopentylpyruvate is evenly dissolved in phosphoric acid in salt buffer;
[0035] (2) Add amino donor: Add 11.21kg of L-glutamic acid as amino donor to the 200L reactor, stir until completely dissolved, and adjust the system to pH=8.0;
[0036] (3) Add aminotransferase and coenzyme: Add 0.025kg coenzyme pyridoxal phosphate PLP and 0.12kg aminotransferase main enzyme BcATen to the 200L reactor;
[0037] (4) Reaction: The system was stirred in the reaction kettle at a stirring speed of 200rpm, and kept at 25°C for 60h;
[0038] (5) Post-treatment: system sampling tracking, raw material conversion is completed, add concentrated...
Embodiment 3
[0040] Embodiment 3: the method for utilizing aminotransferase to synthesize chiral cyclic alkyl amino acid is characterized in that the specific steps are as follows:
[0041] (1) Feeding: Add 1kg of main raw material cycloheptylpyruvate to the 200L reactor Add 100L phosphate buffer (50mM, pH=7.5) to make the main raw material cycloheptylpyruvate Uniformly disperse in phosphate buffer, then add 0.71kg NaOH to make sodium salt, and evenly dissolve in phosphate buffer;
[0042] (2) Add amino donor: Add 1.56kg of L-aspartic acid as amino donor to the 200L reactor, stir until completely dissolved, and adjust the system to pH=7.5;
[0043] (3) Add aminotransferase and coenzyme: Add 0.1kg of coenzyme pyridoxal phosphate PLP and 0.5kg of aminotransferase main enzyme AAT into the 200L reactor;
[0044] (4) Reaction: The system was stirred in the reaction kettle at a stirring speed of 250rpm, and kept at 30°C for 48h;
[0045] (5) Post-treatment: system sampling and tracking, aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com