Cyclic olefin ring-opening polymer, hydride thereof, composition of the hydride, and tricyclopentadiene
A technology of tricyclopentadiene and cyclic olefin, which is applied in the field of cyclic olefin ring-opening polymers, can solve the problems of reduced solvent solubility and gel generation, and achieves excellent polymerization conversion rate, refractive index and Abbe number. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
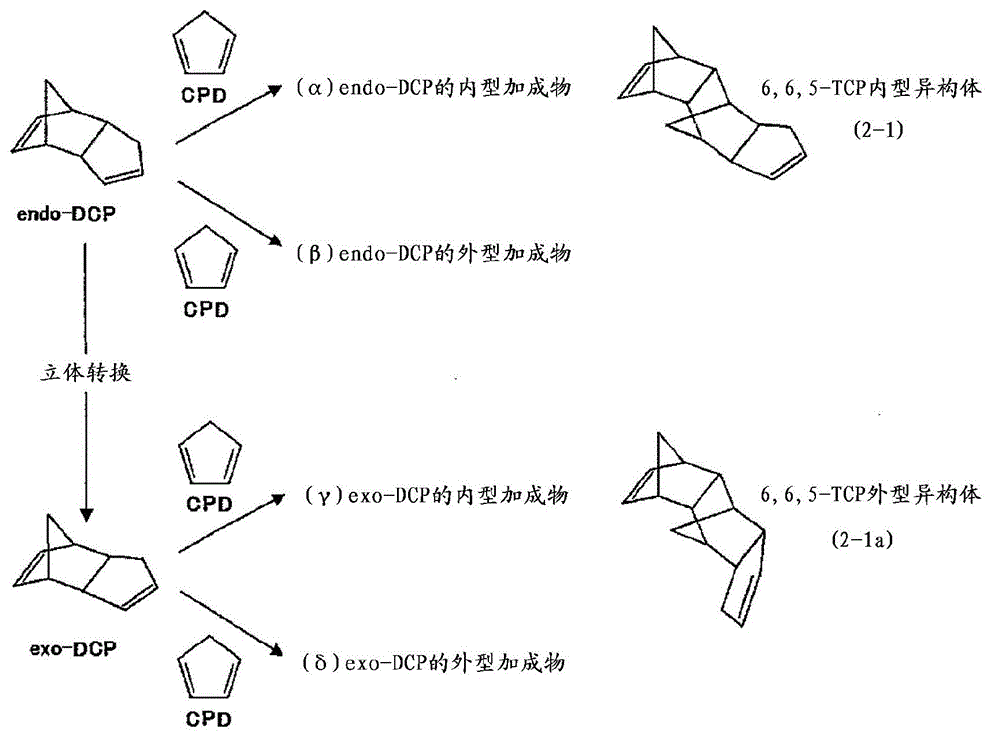
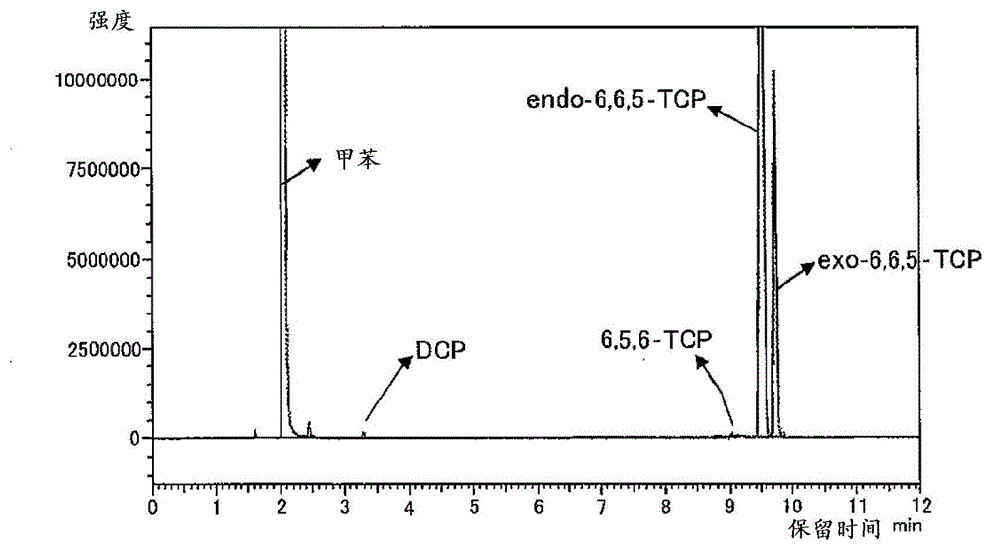
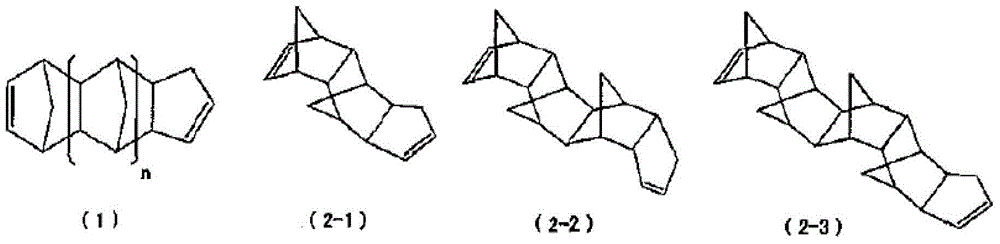
[0246] Add to toluene (275g) the compound represented by formula (2-1) containing 82% of its geometric isomers (including enantiomers, hereinafter, also referred to as compound (2- 1) TCP (71.9g, 363mmol, the content of geometric isomers other than compound (2-1) is 18%), 8-methyl-8-methoxycarbonyl-tetracyclo[4.4.0.1 2,5 .1 7,10 ] Dodec-3-ene (37.5g, 161mmol, hereinafter, also referred to as DNM), bicyclo[2.2.1] hept-2-ene (22.1g, 235mmol, hereinafter, also referred to as NB) and as a molecular weight regulator Add 1-butene (1.69g, 30.1mmol) as the solvent, and heat and stir at 105°C. Separately prepare to mix i-Bu at room temperature 3 Toluene solution (0.28 mL) of Al (75 μmol) and methanol (11 μmol) and WCl 6 (38 μmol) in toluene (0.75 mL). To the toluene solution of the above-mentioned monomers, add i-Bu in sequence 3 Mixed toluene solution of Al and methanol, WCl 6 solution in toluene to start the polymerization reaction. After 1 hour of polymerization, LiOH (228 mi...
Embodiment 2
[0249] As TCP, except for using a substance containing 90% of the compound (2-1) (including enantiomers) among its geometric isomers, the same operation as in Example 1 was carried out to obtain ring-opening copolymerization hydrogenation things [2]. pass 1 The hydrogenation rate analyzed by H-NMR was 99.9%. Tg = 139°C by DSC. As a result of GPC measurement, the weight average molecular weight (Mw) = 36000, and the molecular weight distribution (Mw / Mn) = 2.8. In addition, the refractive index n D =1.533, the Abbe value is 57. The results are shown in Table 1.
Embodiment 3
[0251] As TCP, except for using a substance containing 96% of compound (2-1) (including enantiomers) among its geometric isomers, the same operation as in Example 1 was carried out to obtain ring-opening copolymerization hydrogenation things [3]. pass 1 The hydrogenation rate analyzed by H-NMR was 99.9%. Tg = 139°C by DSC. As a result of GPC measurement, the weight average molecular weight (Mw) = 40000, and the molecular weight distribution (Mw / Mn) = 2.9. In addition, the refractive index n D =1.533, the Abbe value is 56. The results are shown in Table 1.
[0252] [Comparative Example 1]
[0253]As TCP, using a geometric isomer containing 50% of the compound (2-1) (including enantiomers), the same operation as in Example 1 was carried out to obtain a ring-opening copolymerization hydride [4]. pass 1 The hydrogenation rate analyzed by H-NMR was 99.9%. Tg = 140°C as measured by DSC. As a result of GPC measurement, the weight average molecular weight (Mw) = 39000, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com