Bilastine crystal and preparation method thereof
A crystal form, benzodiimazole technology, applied in the field of medicine, achieves good stability and is not easy to crystallize.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1.0 g of 4-[2-[1-(2-ethoxyethyl)-1H-benzodiimazol-2-yl]-1-piperidinyl]ethyl]-α,α-dimethyl Phenylacetic acid was added to 8ml of methanol, heated to dissolve, refluxed for one hour, naturally cooled and crystallized, filtered, and dried in vacuum at 50°C for 10 hours to obtain 0.72 grams of 4-[2-[1-(2-ethoxyethyl) -1H-Benzodeimazol-2-yl]-1-piperidinyl]ethyl]-α,α-Dimethylphenylacetic acid new crystal form, the yield is 72%, and the measured melting point is 197-200°C .
Embodiment 2
[0018] 2.0 g of 4-[2-[1-(2-ethoxyethyl)-1H-benzodiimazol-2-yl]-1-piperidinyl]ethyl]-α,α-dimethyl Add phenylacetic acid to 16ml of toluene, heat to dissolve, keep the temperature at about 70°C, keep it warm for one hour, cool and crystallize naturally, filter, and dry in vacuum at 50°C for 10 hours to obtain 1.5 grams of 4-[2-[1-(2 -Ethoxyethyl)-1H-benzodiimazol-2-yl]-1-piperidinyl]ethyl]-α,α-Dimethylphenylacetic acid new crystal form, yield 75%, measured The melting point is 197.5-200.2°C.
Embodiment 3
[0020] 1.5 g of 4-[2-[1-(2-ethoxyethyl)-1H-benzodiimazol-2-yl]-1-piperidinyl]ethyl]-α,α-dimethyl Add phenylacetic acid to 14ml of ethyl acetate, heat to dissolve, keep the temperature at about 65°C, keep it warm for one hour, cool and crystallize naturally, filter, and dry in vacuum at 50°C for 10 hours to obtain 1.0 g of 4-[2-[1- New crystal form of (2-ethoxyethyl)-1H-benzodiimazol-2-yl]-1-piperidinyl]ethyl]-α,α-dimethylphenylacetic acid, yield 66.7% , and the measured melting point is 197.5-199.8°C.
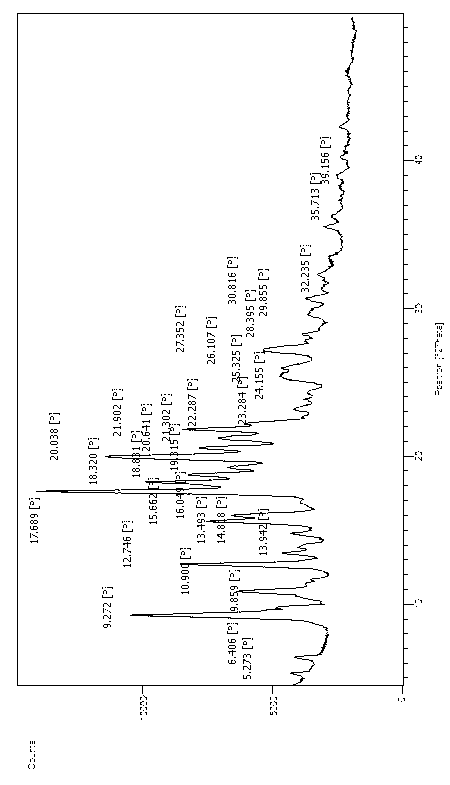
[0021] attached figure 1 X-powder diffraction pattern of bilastine crystal form
[0022] degree 2θ d-spacing Relative Strength(%) 9.2717 9.54 69.73 10.9004 8.11 35.37 12.7461 6.94 53.88 15.6617 5.65 45.34 17.6890 5.01 100.00 18.3202 4.84 64.71 20.0376 4.43 77.25 21.9021 4.05 52.89 27.3518 3.26 26.65
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com