Anti-angiogenesis therapy for the treatment of ovarian cancer
A technology for ovarian cancer and therapy, applied in the direction of anti-growth factor immunoglobulin, antibody, anti-tumor drugs, etc., can solve the problem of high mortality rate of cases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
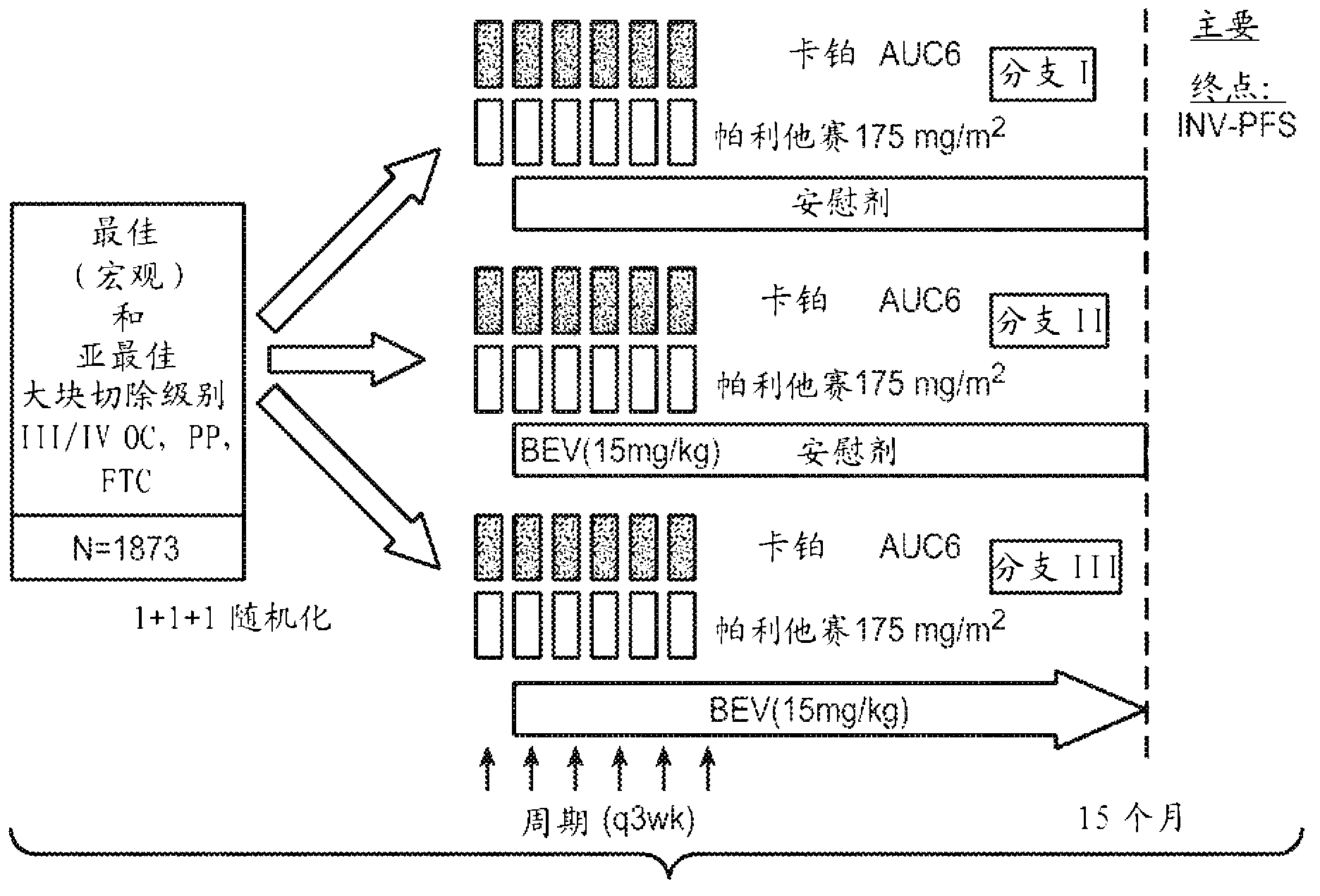
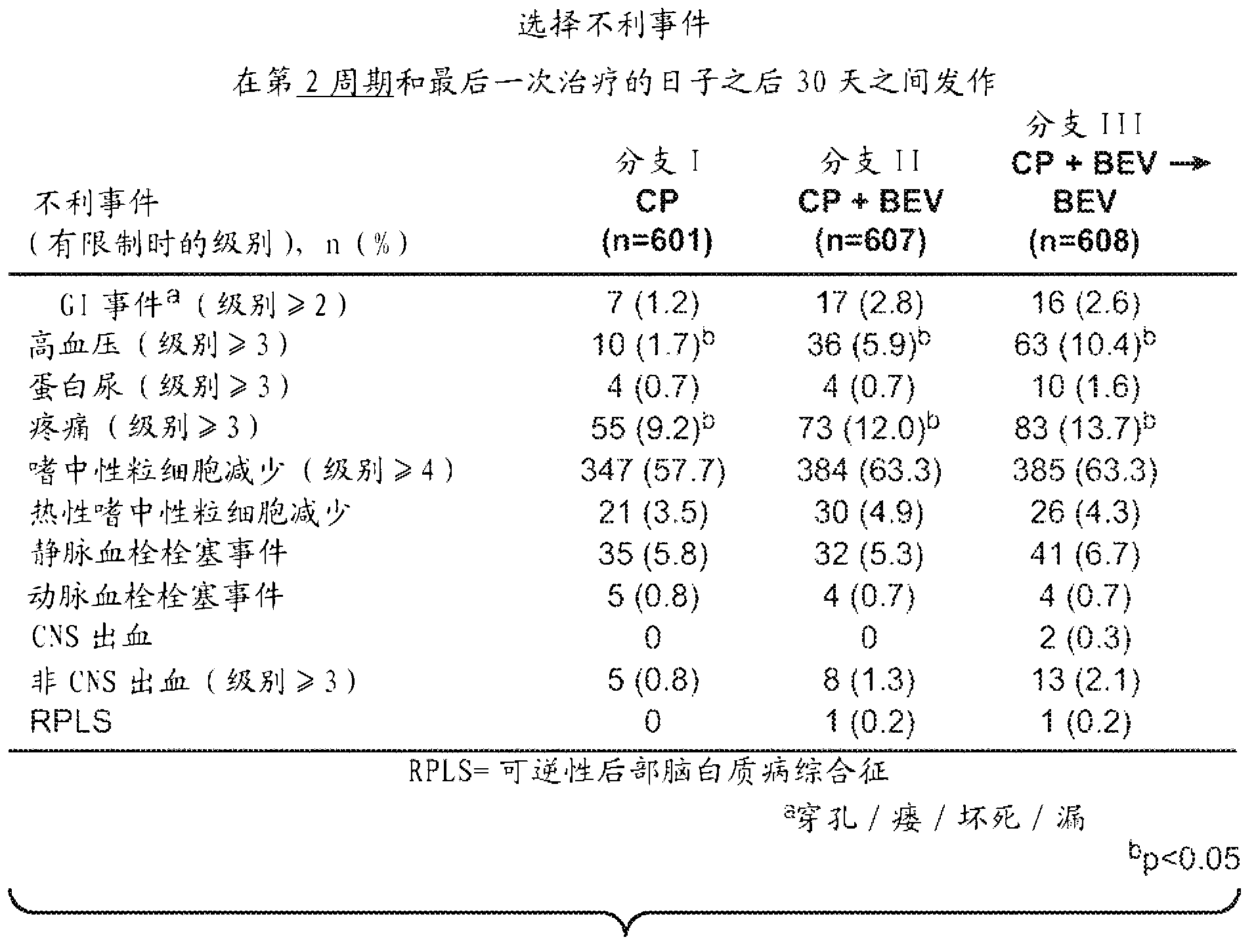
[0255] Example 1: Carboplatin and Paclitaxel plus Placebo vs. Carboplatin and Paclitaxel plus Concurrent Bevacizumab, followed by Placebo, Versus Carboplatin and Paclitaxel plus Concurrent and Extended Bevacizumab Anti, stage III in women with newly diagnosed, previously untreated, stage III (suboptimal and macroscopically optimal massively resected) or IV epithelial ovarian, primary peritoneal, or fallopian tube cancer test
[0256] Presents results from a phase III randomized study of patients with International Federation of Obstetrics and Gynecologic Oncology (FIGO) stages III and IV epithelial ovarian, primary peritoneal or fallopian tube cancer (epithelial ovarian, peritoneal primary or fallopian tube cancer). cancer) to evaluate new treatment procedures. The primary objective included determining the addition of 5 concurrent cycles of bevacizumab to 6 in women with newly diagnosed stage III (with any overall residual disease) and stage IV epithelial ovarian, primary pe...
Embodiment 2
[0310] Example 2. A randomized, two-arm, multicenter gynecologic cancer intergroup trial of adding bevacizumab to standard chemotherapy (carboplatin and paclitaxel) in patients with epithelial ovarian cancer
[0311] Results from a phase III randomized study (ICON7) to assess the safety and efficacy of adding bevacizumab to standard chemotherapy of carboplatin and paclitaxel are presented. The primary endpoint was determined in patients with newly diagnosed, histologically confirmed, high-risk International Federation of Gynecology and Obstetrics (FIGO) stages I and IIa (grade 3 or only clear cell carcinoma) and FIGO stages IIb-IV (all grades and All histologic types) epithelial ovarian, fallopian tube, or primary peritoneal cancer, who have undergone primary surgery (massive excisional cytoreductive surgery or biopsy if patient has FIGO stage IV disease) and will not be diagnosed before disease progression Whether the addition of bevacizumab to standard chemotherapy improves...
Embodiment 3
[0402] Example 3. A phase III, multicenter, randomized, blinded, placebo-controlled trial of carboplatin and gemcitabine plus bevacizumab in patients with platinum-sensitive recurrent ovarian, primary peritoneal, or fallopian tube cancer
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com