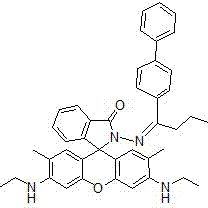
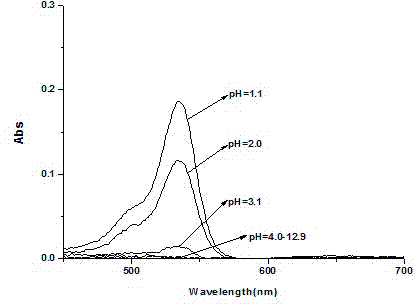
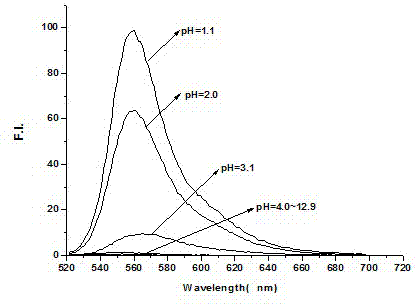
Rhodamine 6GpH fluorescent molecular probe containing biphenyl group as well as preparation method and application thereof
A technology of biphenyl and butyryl biphenyl is applied in the application field of chemical sensing technology, which can solve many problems and achieve the effect of high pH response sensitivity and narrow pH response range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] compound Synthesis
[0033] (1) Synthesis of raw material rhodamine 6G hydrazide
[0034] Add 2.0 g of Rhodamine 6G into a 100 mL flask, then add 20 mL of ethanol to dissolve the solid, add 3.2 mL of 80% hydrazine hydrate to the mixed system at room temperature, stir for 30 minutes, then heat the mixture to reflux, when the solution turns from scarlet to When it turned into a clear orange color, the reaction was complete, cooled to room temperature, rotary evaporated, and the solvent was removed to obtain 1.7 g of the product with a yield of 87.5%.
[0035] (2) Synthesis of the target compound
[0036] Add 1.0 g of rhodamine 6G hydrazide and 0.4 g of butyrylbiphenyl into a 100 mL flask, then add 6 mL of methanol to dissolve the solid, then add dropwise 0.1 mL of glacial acetic acid as a catalyst, heat the mixed system to reflux until complete reaction, wait After the reaction was completed, it was lowered to room temperature, and the mixed system in the flask was po...
Embodiment 2
[0043] compound Synthesis:
[0044] (1) Synthesis of raw material rhodamine 6G hydrazide
[0045] Add 5.0 g of Rhodamine 6G into a 250 mL flask, then add 100 mL of ethanol to dissolve the solid, add 8 mL of 80% hydrazine hydrate to the mixed system at room temperature, stir for 30 minutes, then heat the mixture to reflux, when the solution turns from scarlet When it turned into a clear orange, the reaction was complete, cooled to room temperature, and the solvent was removed by rotary evaporation to obtain 3.9 g of the product with a yield of 81.6%.
[0046] (2) Synthesis of the target compound
[0047] Add 2.5 g of rhodamine 6G hydrazide and 1.1 g of butyrylbiphenyl into a 100 mL flask, then add 15 mL of methanol to dissolve the solid, then add 0.25 mL of glacial acetic acid as a catalyst, heat the mixed system to reflux until complete reaction, wait After the reaction was completed, it was lowered to room temperature, and the mixed system in the bottle was poured into a ...
Embodiment 3
[0049] compound Synthesis:
[0050] (1) Synthesis of raw material rhodamine 6G hydrazide
[0051] Add 10.0 g of rhodamine 6G into a 500 mL flask, then add 200 mL of ethanol to dissolve the solid, add 16 mL of 80% hydrazine hydrate to the mixed system at room temperature, stir for 30 minutes, then heat the mixture to reflux, when the solution turns from scarlet When it turned into a clear orange, the reaction was complete, cooled to room temperature, and the solvent was removed by rotary evaporation to obtain 7.0 g of the product with a yield of 72.8%.
[0052] (2) Synthesis of the target compound
[0053] Add 5.0 g of rhodamine 6G hydrazide and 2.2 g of butyrylbiphenyl into a 100 mL flask, add 30 mL of methanol to dissolve the solid, then add 0.5 mL of glacial acetic acid as a catalyst, heat the mixed system to reflux until complete reaction, wait After the reaction was completed, it was lowered to room temperature, the mixed system in the flask was poured into a beaker fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com