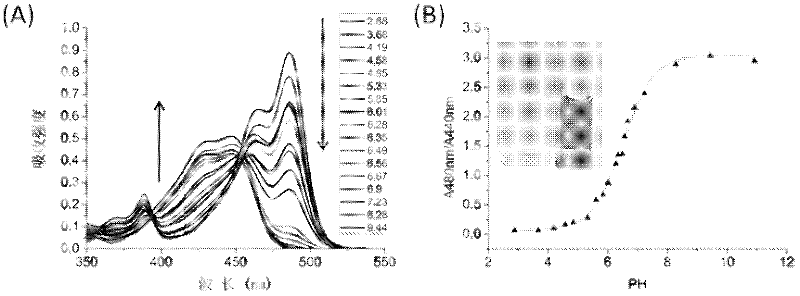
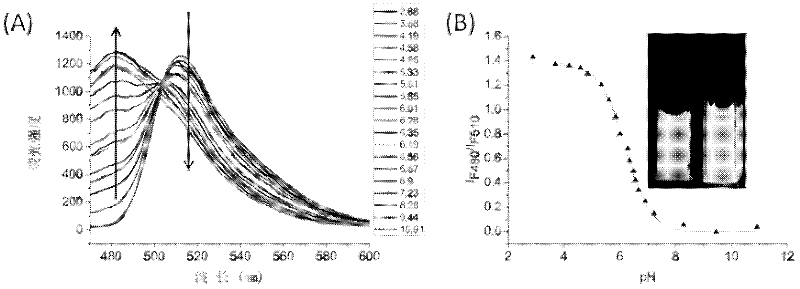
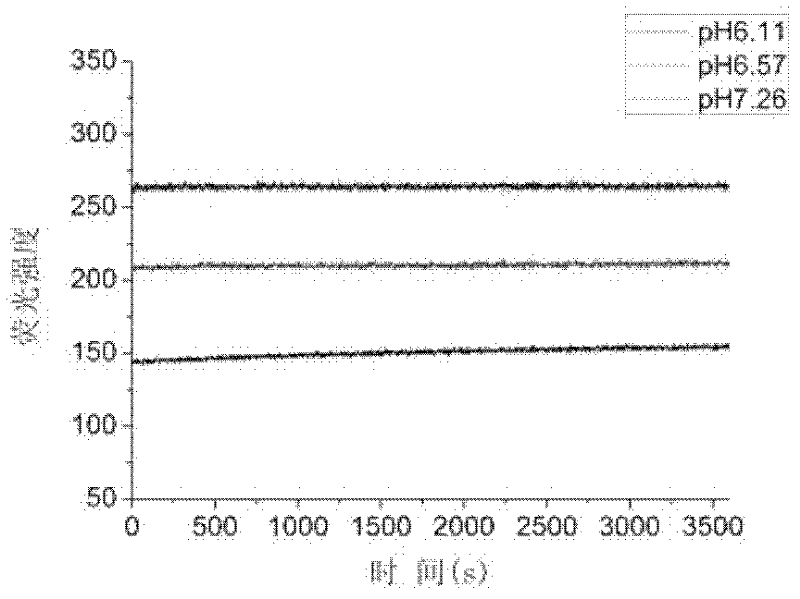
Heterocyclic-fused naphthalimide and preparation method and application thereof
A reaction, carbon atom technology, applied in the field of fluorescent dyes, can solve the problems that the accuracy of detection is easily affected by the change and fluctuation of probe concentration, and the detection range is wide, and achieves good chemical/light stability, high yield, specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, the synthesis of heterocyclic naphthalimide (ENNA)
[0047] Heterocyclic naphthalimide (ENNA) (R 1 for -(CH 2 ) 2 OH,R 2 ,R 3 ,R 4 ,R 5 or R 6 Both are H), the chemical name is: N-hydroxyethyl-4H-1,2,4-triazole (1,5-a) diazacyclo-1,8-naphthalimide synthetic route is as follows :
[0048]
[0049] 1. N-hydroxyethyl-4-bromo-1,8-naphthalimide (B 1 )Synthesis
[0050]
[0051] Add 2g (7.22mmol) 4-bromo-1,8-naphthalic anhydride (A) into a 250ml single-necked round bottom bottle, add 160ml of ethanol and heat to reflux. After heating and stirring for a while, cool to 50°C, slowly add 440 μl of ethanolamine (7.3 mmol) in 5 ml of ethanol solution dropwise, heat up to reflux after dripping, the cloudy solution becomes clear at once, continue to reflux for 1 hour, the reaction ends, and solids precipitate out after cooling , suction filtration, the solid was washed three times with water and ethanol, and dried to obtain compound B (off-white solid B...
Embodiment 2
[0059] Embodiment 2, the synthesis of heterocyclic naphthalimide (BNNA)
[0060] And heterocyclic BNNA (R 1 for -(CH 2 ) 3 CH 3 ,R 2 ,R 3 ,R 4 ,R 5 or R 6 Both are H) chemical name: N-butyl-4H-1,2,4-triazole (1,5-a)diazacyclo-1,8-naphthalimide, the synthetic route is as follows:
[0061]
[0062] 1. N-butyl-4-bromo-1,8-naphthalimide (B 2 )Synthesis
[0063]
[0064] Add 1.5g (5.42mmol) of 4-bromo-1,8-naphthalic anhydride (A) into a 250ml single-necked round bottom bottle, add 120ml of ethanol and heat to reflux. After heating and stirring for a while, cool to 50°C, slowly add 400 μl n-butylamine (5.6 mmol) in 5 ml ethanol solution dropwise, and heat up to reflux after dropping, the turbid solution becomes clear at once, the color deepens, turns orange, and continues to reflux After 40 minutes, the reaction was completed, and the precipitate was precipitated by adding water, filtered by suction, washed three times with water and ethanol respectively, and dried ...
Embodiment 3
[0074] Embodiment 3, the synthesis of heterocyclic naphthalimide (HNNA)
[0075] And heterocyclic HNNA (R 1 for -(CH 2 ) 5 COOH, R 2 ,R 3 ,R 4 ,R 5 or R 6 Both are H): N-hexanoyl-4H-1,2,4-triazole (1,5-a)diazacyclo-1,8-naphthalimide synthetic route is as follows:
[0076]
[0077] 1. N-hexanoyl-4-bromo-1,8-naphthalimide (B 3 )Synthesis
[0078]
[0079] Add 1.5g (5.42mmol) of 4-bromo-1,8-naphthalene dicarboxylic anhydride (A) in a 250ml single-port round bottom bottle, and 0.74mg of 6-aminocaproic acid (5.6mmol), then add 100ml of ethanol and heat to In the reflux state, when the reaction solution changes from the initial milky white turbid liquid to a brown clear liquid, the reaction is completed, cooled, added water to precipitate the precipitate, filtered with suction, washed the solid with water and ethanol three times, and dried to obtain compound B 3 (off-white solid B 3 ), weighing 1.08g, yield 50.9%, the temperature of the above-mentioned reflux is 90 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com