Environment-friendly novel method for preparing primary alcohol from furan or tetrahydrofuran derivatives
A technology of tetrahydrofuran and derivatives, applied in the field of catalytic chemistry, can solve the problems of expensive ionic liquids, high production costs, and complicated separation, and achieve good industrial prospects, reduce separation costs, and facilitate separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
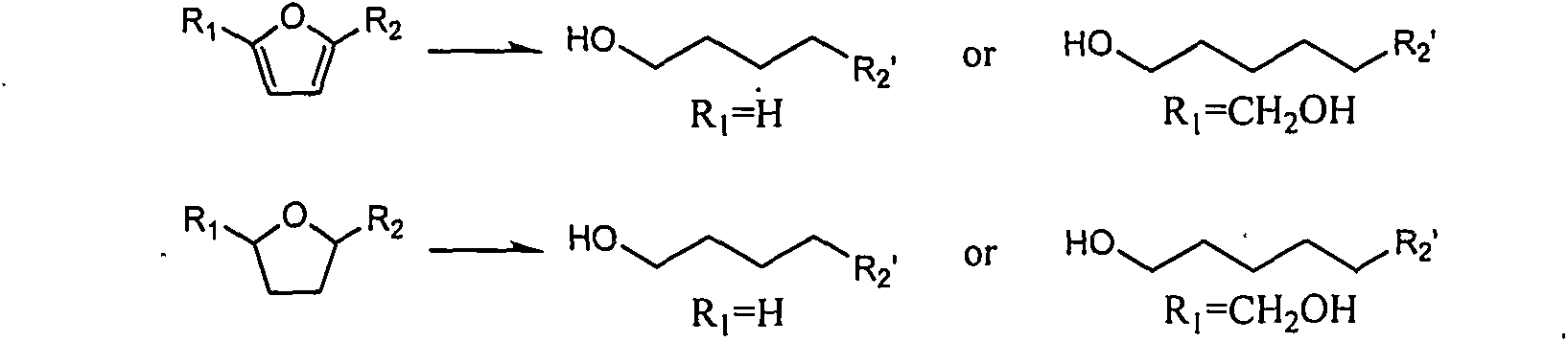
[0013] Examples 1-10 use the multifunctional catalyst Pd / H-ZSM-5 to catalyze different reaction substrates and react under different reaction conditions. The reaction substrates and corresponding primary alcohols are shown in Table 1.
[0014] Table 1
[0015] Example
Five-membered heterocycle
R1
R2
R 2 ′
Example 1
-H
-CHO
-CH 3
Example 2
-H
-CH=CHCOCH 3
-CH 2 CH 2 CH 2 CH 3
Example 3
-H
-CH 2 COHCH 3
-CH 2 CH 2 CH 3
Example 4
-H
-CH 2 CH 3
-CH 2 CH 3
Example 5
-H
-CH=CHCOCH=CH-C 4 H 3 O
-(CH 2 ) 9 -OH
Example 6
Furan
-CH 2 OH
-CHO
-CH 3
Example 7
-CH 2 OH
-CH 2 CH 2 COOH
-CH 2 CH 2 CH 3
Example 8
Tetrahydrofuran
-CH 2 OH
-CH 2 CH 2 -C 6 H 5
-CH 2 CH 2 -C 6 H 11
Example 9
Furan
-CH 2 OH
-CH 2 CH 2 Cl
-CH 2 CH 2 Cl
Example 10
Tetrahydrofuran
-CH 2 OH
-CH 2 COOCH 3
-CH 2 CH 3
[0016] Example 1-10 reaction conditions and reaction results are shown in Table 2
[0...
Embodiment 11-20
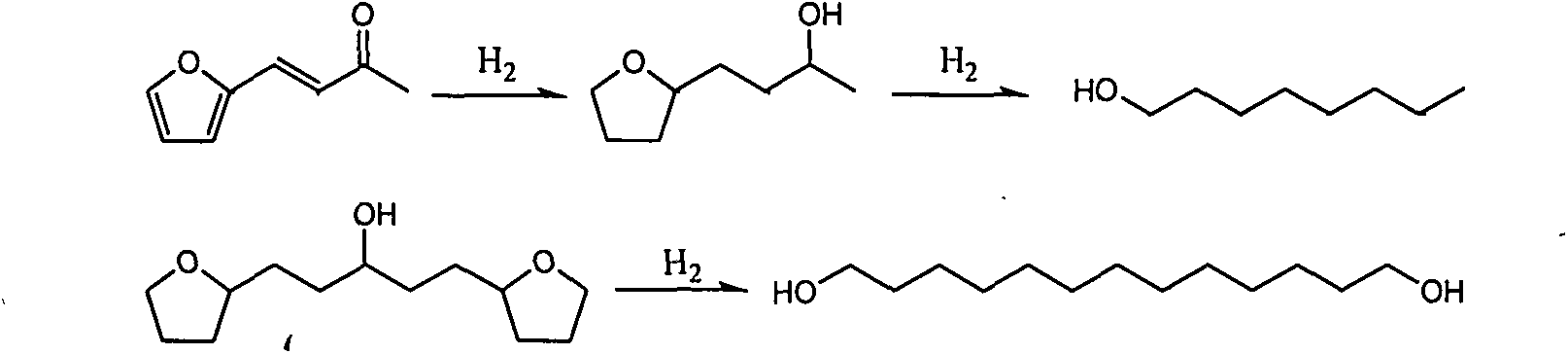
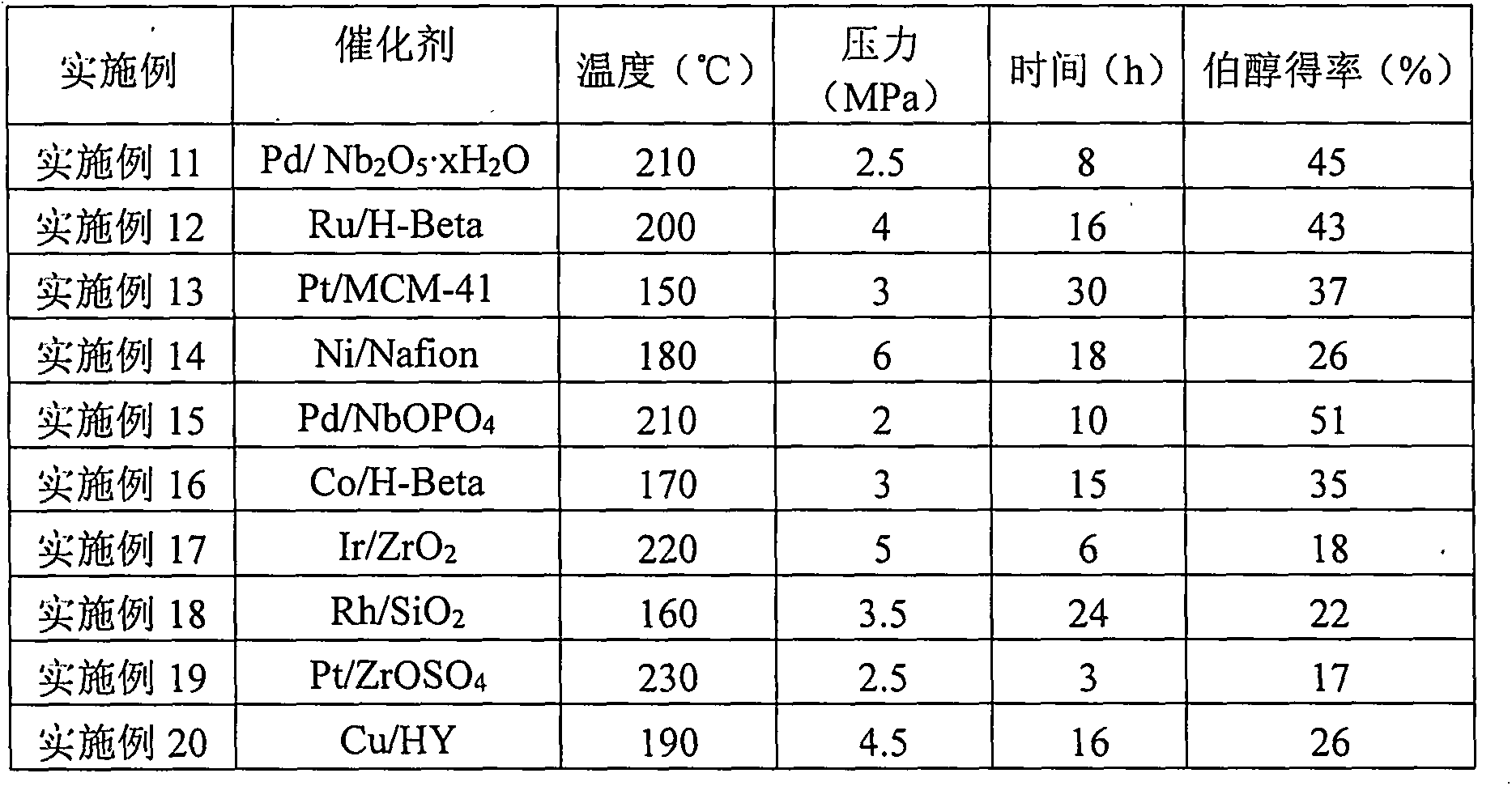
[0020] Examples 11-20 select 4-(2-furyl)-3-buten-2-one as the substrate, select different multifunctional catalysts to catalyze, and react under different conditions. The reaction results are shown in Table 3.
[0021] table 3
[0022]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com