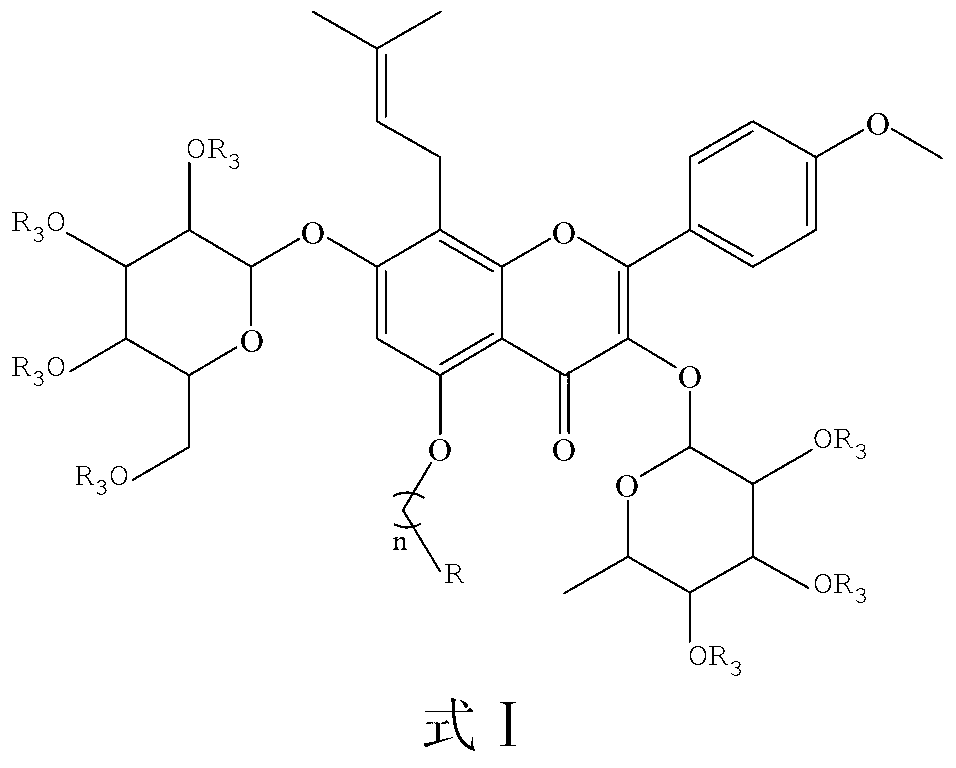
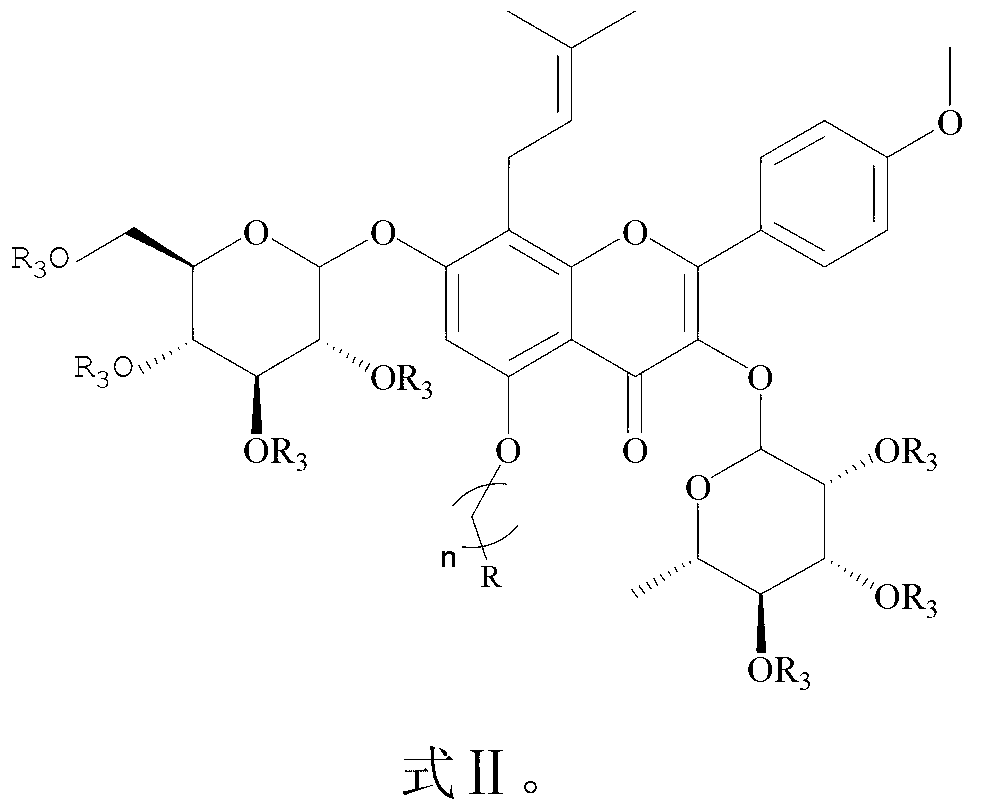
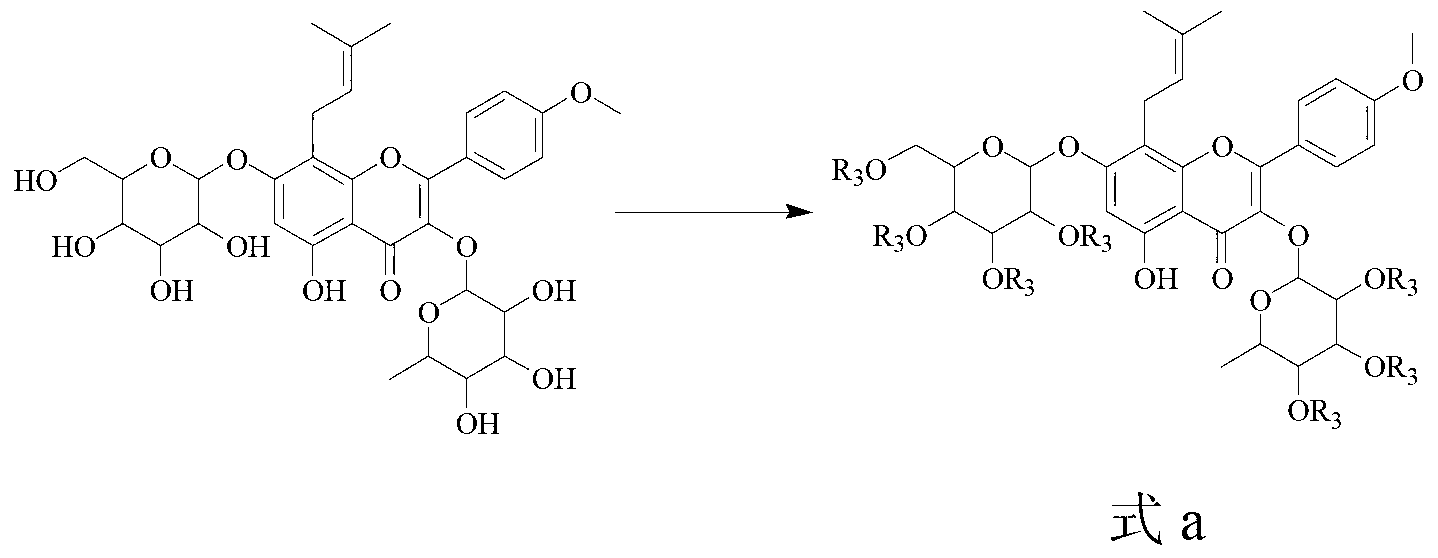
Icariin derivatives
A technology of icariin and derivatives is applied in the directions of sugar derivatives, sugar derivatives, sugar derivatives preparation, etc., to achieve the effect of excellent medicinal effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of Icariin Derivatives (Compounds 7, 8) of the present invention
[0036] Dissolve 5 g (7.4 mmol, 1 equiv) of ICA in 30 mL of DMF, add 5.7 g (51.8 mmol, 7 equiv) of triethylamine in an ice bath, stir for 10 min, add 5.7 g (51.8 mmol, 7 equiv) of acetic anhydride dropwise, and Stirred in the bath for 6h, quenched with ice water, filtered, washed with water, dissolved in ethyl acetate, dried over anhydrous MgSO4, concentrated in vacuo, and column chromatographed to obtain an alcoholic hydroxyl-protected icariin derivative (1 g, 15%). Dissolve 0.5 g (0.52 mmol, 1 equiv) of the protected product in 2 mL of anhydrous acetone, add appropriate amount of anhydrous K2CO3, catalytic amount of KI, stir for 10 min, add 0.55 mmol (1.1 equiv) of 1,3-dibromopropane or Ethyl bromoacetate, stirred at room temperature for 1-3h, and monitored the reaction by TLC. After the reaction was completed, the solid was removed by filtration, and the filtrate was concentrated ...
Embodiment 2
[0039] Example 2 Synthesis of Nitrogenous Derivatives of Icariin (Compounds 9 and 10)
[0040] Take 0.19g (0.17mmol, 1equiv) of compound 7 and dissolve it in anhydrous acetone, add an appropriate amount of K 2 CO 3 and catalytic amount KI, stirred at room temperature for 10 min, added piperidine or piperazine, stirred at room temperature for 30 min, filtered to remove solids, and the filtrate was concentrated in vacuo. The crude product was purified by column chromatography to obtain products 9 and 10.
[0041]Compound 9: Yellow powder, yield 90%.Mp.120~121℃; 1H NMR (400MHz, CDCl3) δ7.82(d, 2H, J=8.80Hz), 7.06(d, 2H, J=8.80Hz) ,6.92(s,1H),5.67(t,1H,J=2.08Hz),5.52(d,1H,J=7.04Hz),5.45(s,1H),5.32~5.40(m,2H),5.23~ 5.03(m, 2H), 5.06(t, 1H, J=6.56Hz), 4.96(t, 1H, J=10.08Hz), 4.64(bs, 1H), 4.39~4.31(m, 4H), 3.90(s ,3H),3.71~3.77(bs,4H),3.38~3.48(m,2H),2.50(d,2H,J=5.12Hz),2.13(s,3H),2.03(s,3H),2.00( d, 6H, J=3.84Hz), 1.68(s, 3H), 1.66(s, 3H), 1.63(s, 15H), 1.22~1.26(m, 3H), 0.81(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com