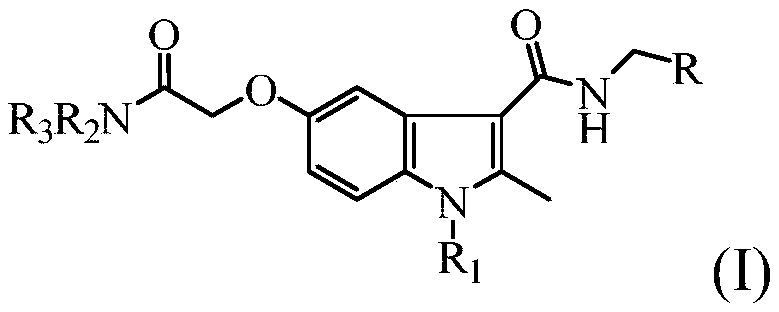
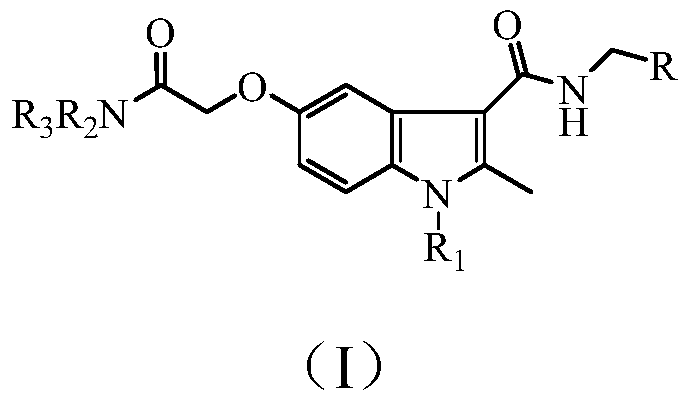
Benzpyrole-3-formamide compound and application thereof
A formamide and compound technology, which is applied in the field of indole-3-carboxamide compounds and their preparation, and antioxidant applications, can solve the problems of reduced ability to scavenge free radicals, and achieve novel structure types, good application value and The effect of developing application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
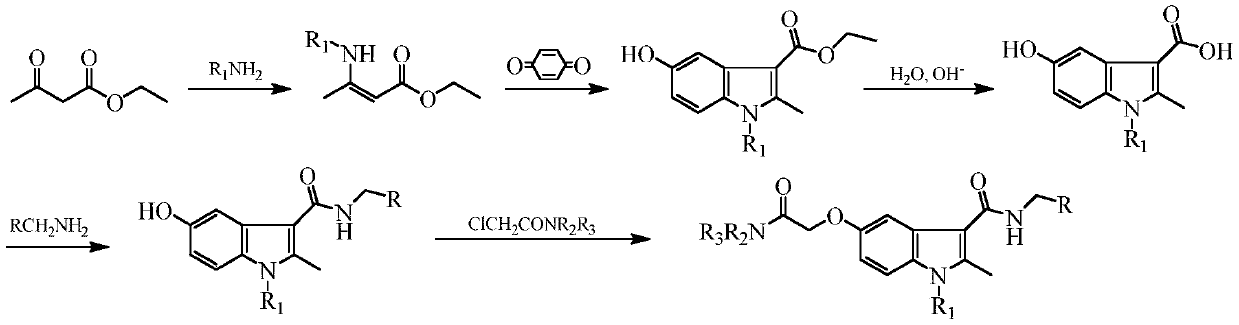
[0023] Example 1: N-benzyl-5-(2-diethylamino-2-oxoethoxy)-1,2-dimethyl-1H-indole-3-carboxamide (compound Z01) preparation
[0024] Step A: Preparation of ethyl 3-methylamino-2-butenoate
[0025] Put ethyl acetoacetate (15.01g, 0.12mol) in a 100mL eggplant-shaped bottle, slowly add 0.36mol of methylamine aqueous solution dropwise under stirring, continue to react for 3h at room temperature after the dropwise addition, let stand to separate layers, and wash the organic phase with water Once, dried over anhydrous sodium sulfate and filtered to obtain 15.08 g of light yellow liquid, yield: 91.4%.
[0026] Step B: Preparation of ethyl 1,2-dimethyl-5-hydroxy-1H-indole-3-carboxylate
[0027] Put p-benzoquinone (11.89g, 0.11mol) and 120mL of acetone in a 250mL eggplant-shaped flask, add 0.11mol of 3-methylamino-2-butenoic acid ethyl ester dropwise under stirring, and control the temperature at 30°C after the dropwise addition The reaction was continued for 2 hours, the solvent was ...
Embodiment 2
[0036] Embodiment 2: N-benzyl-5-(2-diethylamino-2-oxoethoxy)-2-methyl-1-ethyl-1H-indole-3-carboxamide (compound Z02 ) preparation
[0037] Referring to the preparation method of Example 1, 0.62 g of white solid was obtained, yield: 49.2%. M.p.: 105-106℃; IR: (KBr,cm -1 ):υ3434.3, 2925.2, 1661.7, 1617.5, 1530.2, 1478.3, 1347.7, 1259.2, 1189.2, 1139.2, 1082.9, 835.4, 756.0, 704.4; ESI-MS, m / z: calcd.421.24 (M + ); found 422.3([M+H] + ),444.3([M+Na] + ); 1 H NMR (400MHz, CDCl 3 ): δ7.43(d,J=2.3Hz,1H),7.36–7.27(m,5H),7.21(d,J=8.9Hz,1H),6.90(dd,J=8.9,2.4Hz,1H) ,4.71(d,J=5.7Hz,2H),4.65(s,2H),4.12(q,J=7.2Hz,2H),3.36(q,J=7.0Hz,4H),2.72(s,3H) , 1.33 (t, J = 7.2Hz, 3H), 1.17 (t, J = 7.1Hz, 3H), 1.11 (t, J = 7.1Hz, 3H).
Embodiment 3
[0038] Example 3: N-benzyl-5-(2-di-n-propylamino-2-oxoethoxy)-1,2-dimethyl-1H-indole-3-carboxamide (compound Z03) preparation
[0039]Referring to the preparation method of Example 1 Referring to the preparation method of Example 1, 1.08 g of white solid was obtained, yield: 82.4%. M.p.:123-125℃;IR:(KBr,cm -1 ):υ3349.0, 2964.9, 2871.1, 1656.2, 1484.3, 1382.6, 1270.8, 1190.0, 1143.3, 1084.6, 926.2, 839.0, 798.2, 741.2, 697.8; ESI-MS, m / z: calcd.435.25 (M + ); found 436.2([M+H] + ),458.3([M+Na] + ); 1 H NMR (400MHz, CDCl3): δ7.43 (d, J = 2.3Hz, 1H), 7.38–7.26 (m, 5H), 7.20 (d, J = 8.9Hz, 1H), 6.90 (dd, J = 8.9 ,2.4Hz,1H),4.71(d,J=5.7Hz,2H),4.67(s,2H),3.66(s,3H),3.26(q,J=7.0Hz,4H),2.72(s,2H ), 1.56–1.48 (m, 4H), 0.89 (t, J=7.4Hz, 3H), 0.84 (t, J=7.4Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com