Benzimidazole compounds containing ureido group and application
A technology of benzimidazoles and compounds, applied in the field of benzimidazoles and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
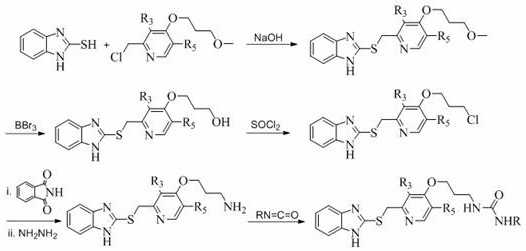
[0035] The schemes outline the preparative steps used to prepare the compounds of the invention.
[0036]
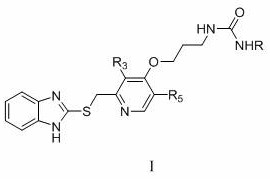
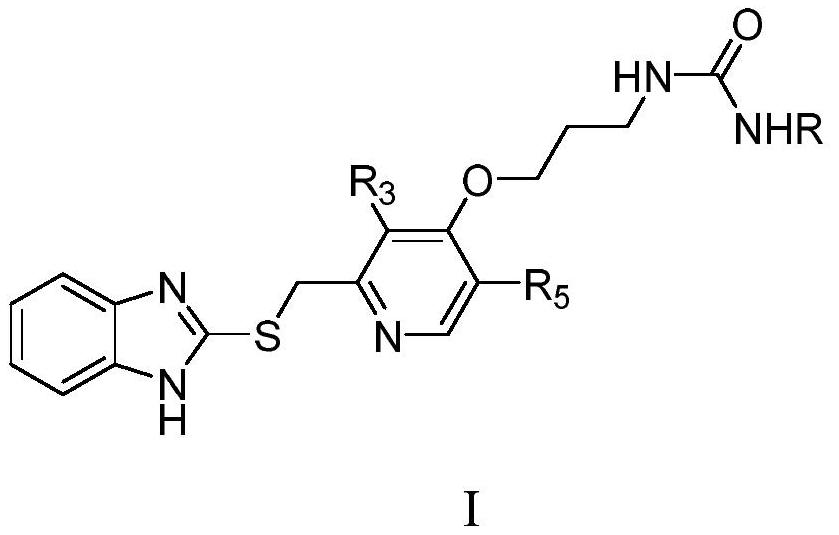
[0037] Among them, R, R 3 , R 5 as mentioned earlier.
[0038] The present invention is described in detail with the following examples. However, it should be understood that the present invention is not limited to the specific recited examples below.
Embodiment 1
[0039] Example 1: 1-{3-{[2-{[(1H-benzimidazol-2-yl)thio]methyl}-3-methyl-pyridin-4-yl}oxy}propyl} -Preparation of 3-phenylurea (compound X01)
[0040] Step A: Preparation of 2-{[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methylthio}-1H-benzimidazole
[0041] 2-Chloromethyl-4-(3-methoxypropoxy)-3-picoline hydrochloride (0.50g, 1.88mmol) was placed in a 125ml eggplant-shaped bottle, and 11ml of ethanol was added to dissolve it, Then 1H-benzimidazole-2-thiol (0.28g, 1.88mmol) and 4ml NaOH (80g / L) were added, refluxed at 68°C for 4h, and the reaction was complete as monitored by TLC. The reaction liquid was poured into a 100ml beaker, cooled naturally to room temperature, a white solid was precipitated, and recrystallized from ethyl acetate and petroleum ether (2:1) to obtain 0.58 g of white needle-like crystals, with a yield of 89.3%. m.p.: 115-118°C (literature value: 116-118°C).
[0042] Step B: Preparation of 3-{{2-{[(1H-benzimidazol-2-yl)thio]methyl}-3-methylpyridin-4-yl}ox...
Embodiment 2
[0048] Example 2: 1-{3-{[2-{[(1H-benzimidazol-2-yl)thio]methyl}-3-methyl-pyridin-4-yl}oxy}propyl} - Preparation of 3-(4-chlorophenyl)urea (compound X02)
[0049] Referring to the preparation method of Example 01, 0.32 g of white solid was obtained with a yield of 76.2%, m.p.: 165.8-167.3°C; ESI-MS: m / z 600.7, 602.0, [M+H] + ; 1 H NMR (400MHz, DMSO-d 6)δ8.23(d,J=5.6Hz,1H),7.59(dt,J=7.2,3.5Hz,1H),7.45(dd,J=6.1,3.1Hz,1H),7.39–7.32(m,2H ),7.23–7.12(m,3H),6.97(d,J=5.7Hz,1H),5.23(s,2H),4.70(s,2H),4.59(t,J=5.1Hz,1H),4.12 (t, J=6.2Hz, 2H), 3.77(d, J=5.0Hz, 2H), 3.62(t, J=4.9Hz, 2H), 3.57(q, J=5.9Hz, 2H), 3.11(d , J=4.9Hz, 2H), 2.98(t, J=5.0Hz, 2H), 2.20(s, 3H), 1.89(p, J=6.2Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com