Compound with PPAR delta agonistic activity, pharmaceutical composition and medical application
A compound and drug technology, applied in the field of compounds with PPARδ agonistic activity, can solve problems such as increasing the risk of fractures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
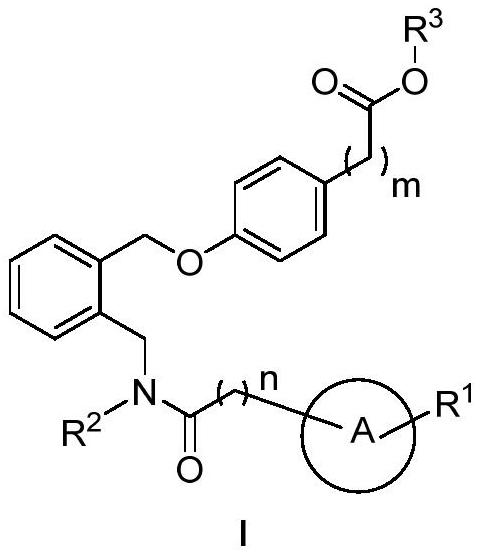
[0062] Ethyl 2-(4-((2-(((N-cyclopropyl-4-(trifluoromethyl)benzamido)methyl)benzyl)oxy)phenyl)acetate (compound 1)
[0063]
[0064] Synthesis of compound I-1
[0065] Under argon protection, 2-cyanobenzyl bromide (300mg, 1.5mmol) was dissolved in anhydrous dichloromethane (5mL), cooled in an ice-water bath, and a 1.5M n-hexane solution of diisobutylaluminum hydride (1.1 mL, 1.6mmol), stirred at 0°C for 4 hours. The reaction solution was poured into cold 48% hydrobromic acid aqueous solution (20 mL), and stirred at room temperature for 1.5 hours. The reaction was monitored by TLC. After the reaction was complete, dichloromethane (20mLx3) was added for extraction, the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure, and the residue was subjected to column chromatography (eluent: petroleum ether / ethyl acetate Ester: 30:1) purification to obtain compound I-1 (yellow green liquid, 217mg, yield: 71%).
[0066...
Embodiment 2
[0073] 2-(4-((2-((N-cyclopropyl-4-(trifluoromethyl)benzamido)methyl)benzyl)oxy)phenyl)acetic acid (compound 2)
[0074]
[0075] Compound 1 (78mg, 0.1mmol) was dissolved in ethanol:tetrahydrofuran (1:1) mixed solvent (2mL), 1N lithium hydroxide aqueous solution (0.3mL) was added, and the reaction was stirred at room temperature. The reaction was monitored by TLC. After the reaction was complete, the solvent was evaporated under reduced pressure, water (10mL) and 1N hydrochloric acid were added successively to adjust the pH to 3, a large amount of white solid was precipitated, filtered with suction, the filter cake was washed with water (10mLx3), and dried in vacuo to obtain the compound 2 (white solid, 50mg, yield: 68%): 1 H NMR (300MHz, DMSO-d 6 )δ12.53-11.87(m,1H),7.84-7.67(m,4H),7.56-7.46(m,1H),7.43-7.36(m,2H),7.37-7.28(m,1H),7.23- 7.17(m,1H),7.18-7.12(m,1H),7.03-6.96(m,1H),6.96-6.90(m,1H),5.17(s,2H),4.80(s,2H),3.49( s,2H),2.87-2.67(m,1H),0.59-0.32(m,4H).MS(ESI):m / z[M+N...
Embodiment 3
[0077] Ethyl 2-(4-((2-(((N-cyclopropyl-4-methoxybenzamido)methyl)benzyl)oxy)phenyl)acetate (Compound 3)
[0078] With reference to the method of Example 1, 4-trifluoromethylbenzoic acid was replaced by 4-methoxybenzoic acid to obtain compound 3: 1 H NMR (300MHz, DMSO-d 6 )δ7.59-7.53(m,1H),7.53-7.49(m,1H),7.50-7.45(m,1H),7.43-7.35(m,2H),7.35-7.27(m,1H),7.24- 7.18(m,1H),7.18-7.14(m,1H),7.02-6.95(m,2H),6.95-6.91(m,2H),5.15(s,2H),4.76(s,2H),4.06( q,J=7.1Hz,2H),3.79(s,3H),3.57(s,2H),2.85–2.74(m,1H),1.17(t,J=7.1Hz,3H),0.57-0.48(m ,2H),0.48-0.41(m,2H).MS(ESI):m / z[M+Na] + 496.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com