Co-M-B amorphous alloy nanotube catalyst, and preparation and application thereof
A technology of amorphous alloys and nanotubes, which is applied in the direction of metal/metal oxide/metal hydroxide catalysts, preparation of hydroxyl compounds, and preparation of organic compounds. Problems such as high particle ratio, to achieve the effect of easy operation and control, improved productivity and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
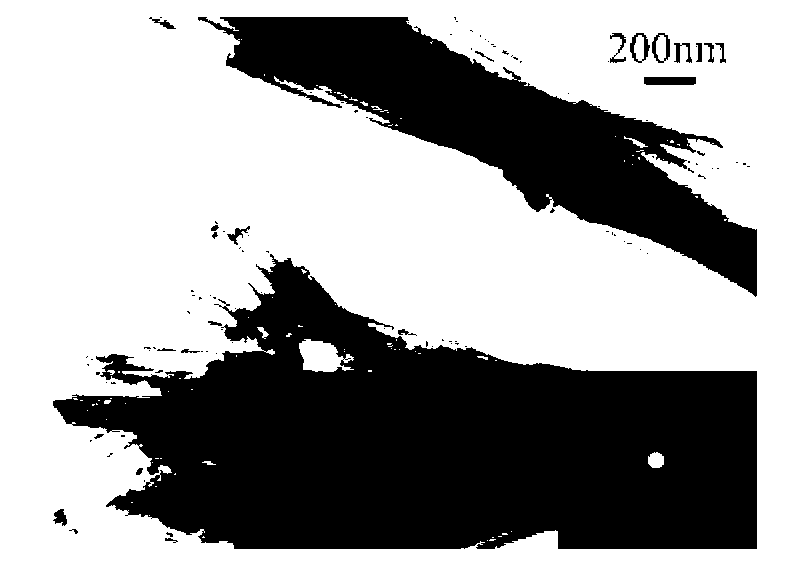
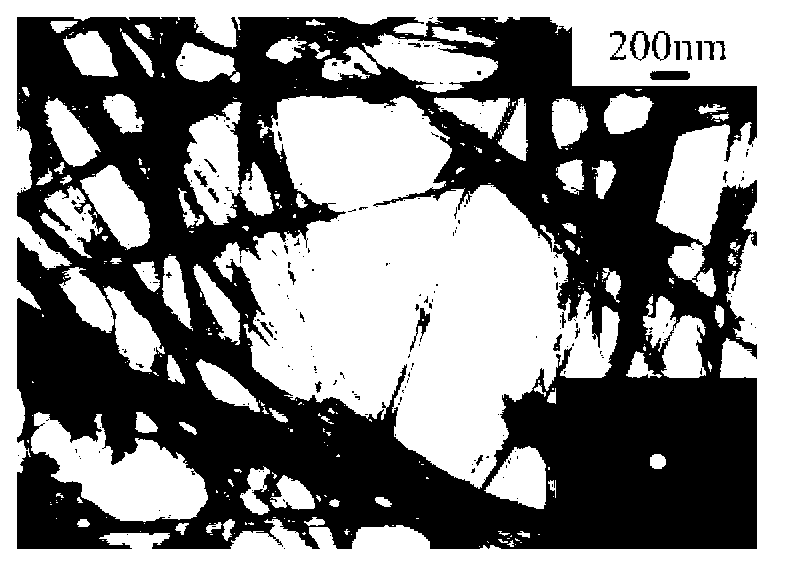
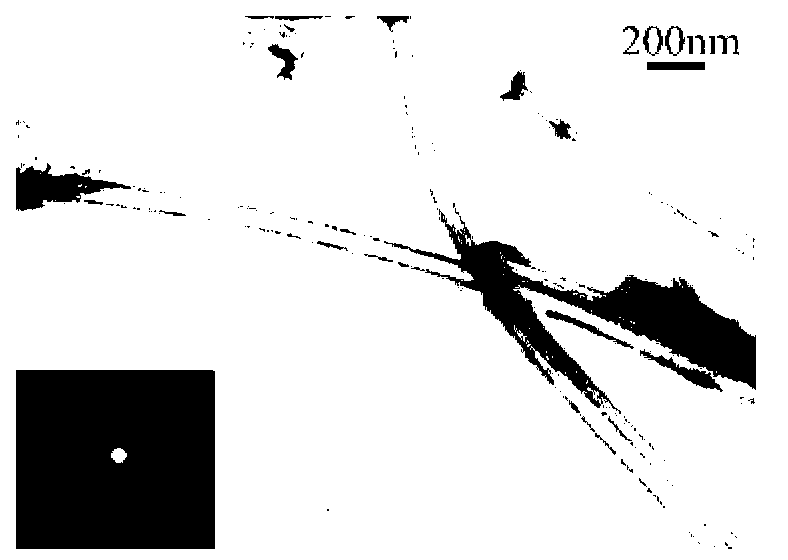
Image
Examples
Embodiment 1
[0068] a) Weigh 0.58g of camphorsulfonic acid, dissolve it in 10mL of deionized water, add 7mL of Tween 60, stir evenly in an oil bath at 55°C to form a transparent solution, and then add 0.44g of molybdic acid to the solution Ammonium and 0.60g cobalt chloride hexahydrate, stirred into a transparent solution, placed in an oil bath at 55°C for 10 minutes;
[0069] b) The above solution is gradually cooled to 25°C to form a liquid crystal precursor, which is transferred to a 250mL three-necked flask to prepare three parts of the liquid crystal precursor, adding 5mL, 10mL and 15mL of acetone solution respectively, and stirring for 10 minute;
[0070] c) Freshly configure 9ml of a mixed aqueous solution of 0.1M sodium hydroxide and 4M sodium borohydride, place the beaker containing the above solution in an ice-water mixing bath, and add it to step b) with a constant flow pump at a flow rate of 0.05mL / min The resulting solution was protected with nitrogen, and the reaction was co...
Embodiment 2
[0075] a) Weigh 0.58g of camphorsulfonic acid, dissolve it in 10mL of deionized water, add 7mL of Tween 60, stir evenly in an oil bath at 55°C to form a transparent solution, and then add 0.62g of partial tungsten to this solution ammonium chloride and 0.60g cobalt chloride hexahydrate, stirred into a transparent solution, placed in an oil bath at 55°C for 10 minutes;
[0076] b) Gradually cool the above solution to 25° C. to form a liquid crystal precursor, transfer the liquid crystal precursor to a 250 mL three-necked flask, add 10 mL of acetone solution, and stir for 10 minutes;
[0077] c) Freshly configure 9ml of a mixed aqueous solution of 0.1M sodium hydroxide and 4M sodium borohydride, place the beaker containing the above solution in an ice-water mixing bath, and add it to step b) with a constant flow pump at a flow rate of 0.05mL / min The resulting solution was protected by argon, and the reaction was complete under magnetic stirring. At room temperature, the above mi...
Embodiment 3
[0081] The difference between this example and Example 1 is that: step b) is to add 10 mL of isopropanol solution.
[0082] All the other contents are the same as those described in Example 1.
[0083] The specific surface area of Co-Mo-B amorphous alloy nanotubes prepared in this example is 117.8m 2 / g, the outer diameter of the nanotube is 65-70nm, the inner diameter is 60-65nm, the thickness of the tube wall is about 3-5nm, and its composition is: Co 68.82 Mo 13.04 B 18.14 (molar ratio); the yield is 92%-95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com