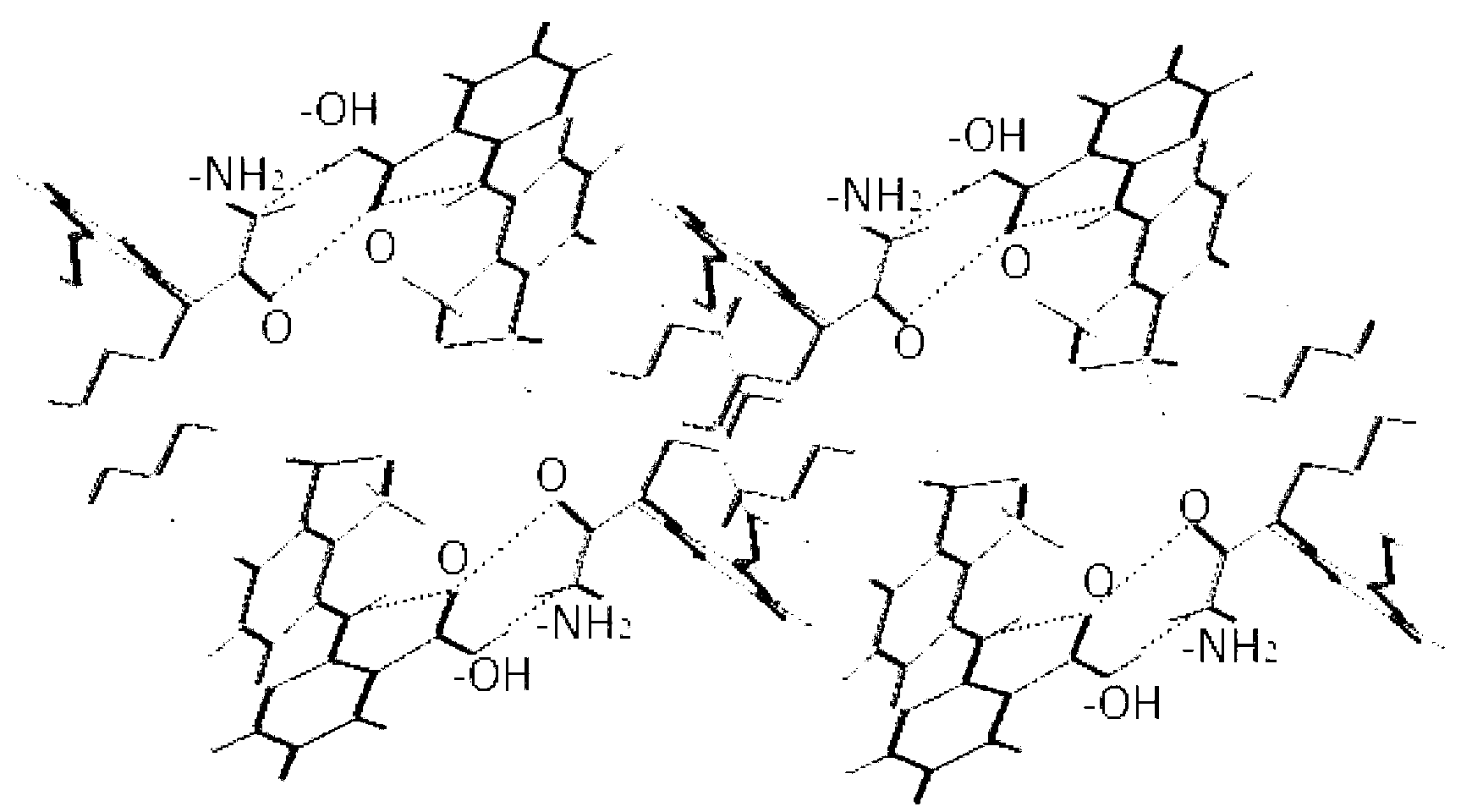
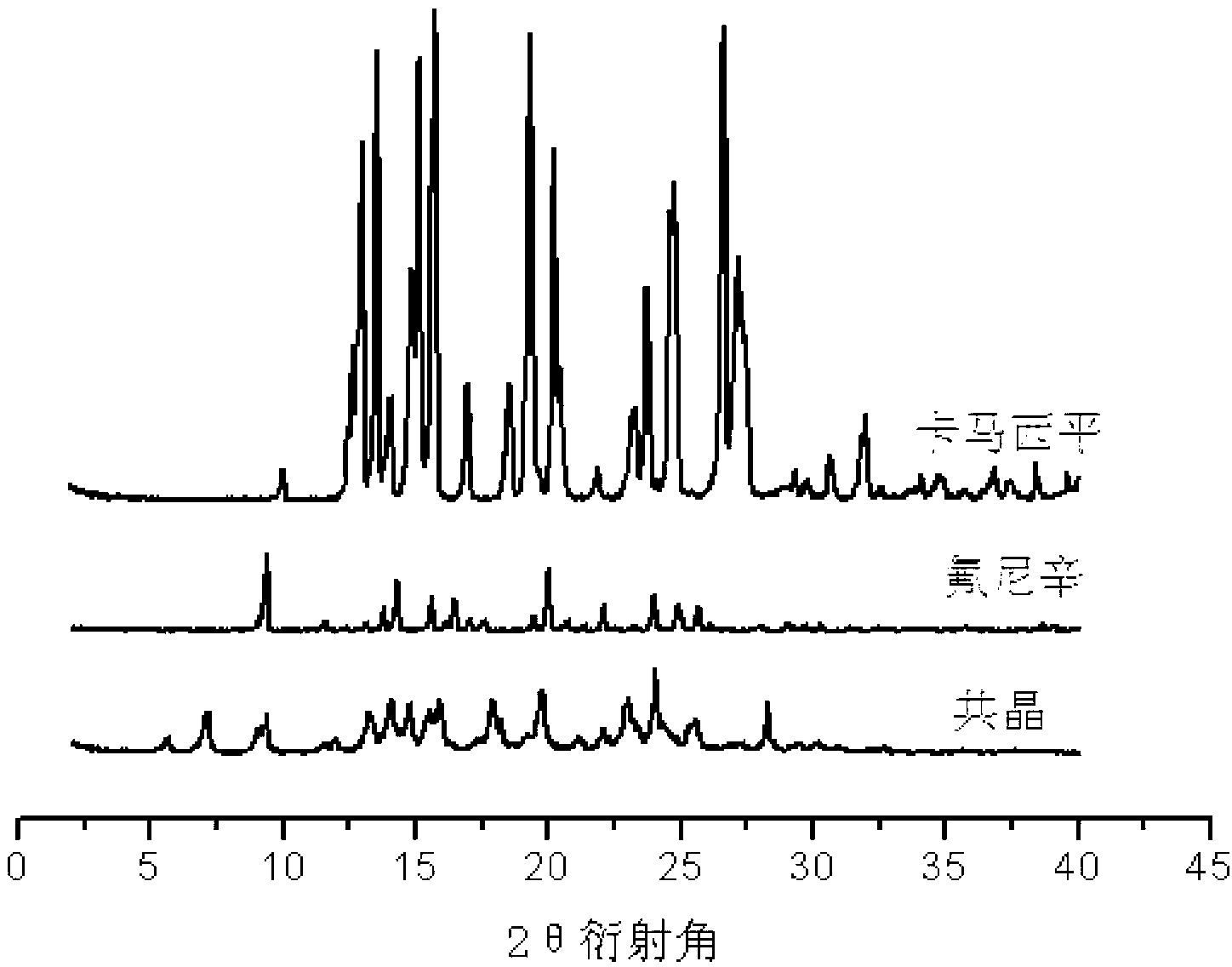
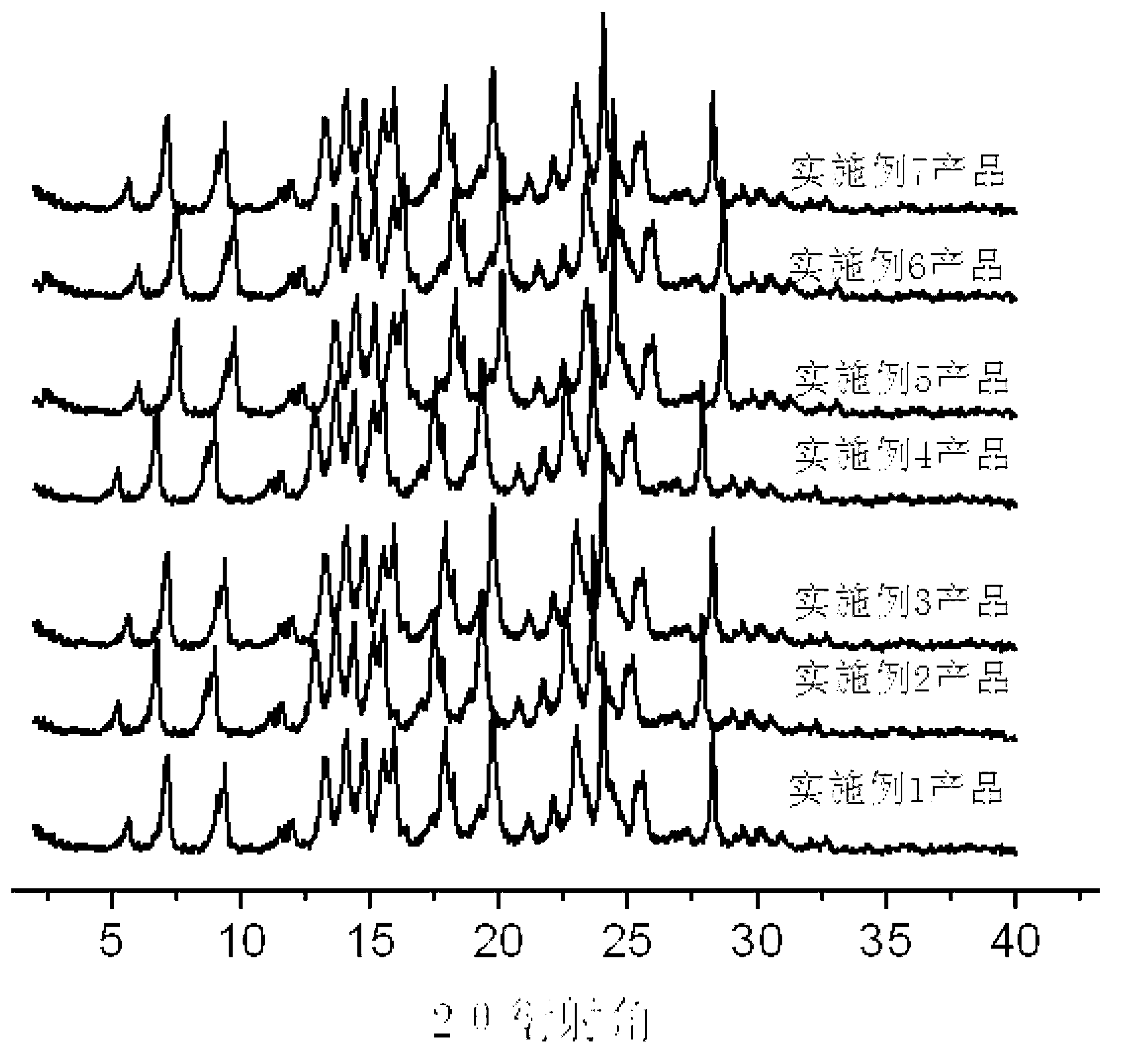
Flunixin and carbamazepine cocrystal and preparation method thereof
A carbamazepine and zepine co-crystal technology, applied in organic chemistry and other directions, can solve the problem of no carbamazepine and other drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of flunixin-carbamazepine eutectic by cooling crystallization:
[0036] 1) Weigh 5.92g of flunixin, 2.36g of carbamazepine, the molar ratio is 2:1, 74.5g of absolute ethanol, the solid accounts for 10% of the total mass of the system, add them to the crystallizer, heat to 52°C, stir and dissolve completely;
[0037] 2) Cool the solution down to 50 minutes after 60 minutes, and grow crystals at a constant temperature of 50°C for 30 minutes;
[0038] 3) The system is cooled to 40°C at 0.05°C / min, and the crystals are filtered
[0039] 4) The crystal was dried in an oven at 50°C for 3 hours to obtain the co-crystal product of flunixin and carbamazepine, with a weighing mass of 3.77 g.
Embodiment 2
[0041] Preparation of flunixin-carbamazepine eutectic by cooling crystallization:
[0042] 1) Weigh 4.44g of flunixin, 3.54g of carbamazepine, the molar ratio is 1:1, 45.2g of absolute ethanol, the solid accounts for 15% of the total mass of the system, add them to the crystallizer, heat to 55°C, and stir to dissolve ;
[0043] 2) The solution was cooled to 50°C after 60 minutes, and the crystal was grown at a constant temperature of 50°C for 30 minutes;
[0044] 3) The system is lowered to 30°C at 1°C / min, and the crystals are filtered;
[0045] 4) The crystals were dried in an oven at 50° C. for 3 hours to obtain the co-crystal product of flunixin and carbamazepine, with a weighing mass of 5.97 g.
Embodiment 3
[0047] Preparation of flunixin-carbamazepine eutectic by cooling crystallization:
[0048] 1) Weigh 5.92g of flunixin, 4.72g of carbamazepine, the molar ratio is 1:1, 42.56g of absolute ethanol, the solid accounts for 20% of the total mass of the system, add them to the crystallizer, heat to 60°C, stir and dissolve ;
[0049] 2) The solution was cooled to 53°C after 40 minutes, and the crystal was grown at a constant temperature of 53°C for 40 minutes;
[0050] 3) The system drops to 45°C at 0.2°C / min, and the crystals are filtered;
[0051] The crystals were dried in an oven at 50° C. for 3 hours to obtain the co-crystal product of flunixin and carbamazepine, with a weighing mass of 7.94 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com