Co-crystal of tapentadol hydrochloride and celecoxib, composition and preparation method thereof
A technology of tapentadol hydrochloride and celecoxib, which is applied in the treatment of pain medicine, the co-crystal of tapentadol hydrochloride and celecoxib and its pharmaceutical composition and preparation field can solve the problem of different concentration Changes in expectations, patient compliance and impairment of treatment benefit, dosing errors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of Tapentadol Hydrochloride and Celecoxib Cocrystal
[0038] Weigh 2.57g of tapentadol hydrochloride and place it in a 100ml round bottom flask, add 10ml of absolute ethanol, stir to dissolve, then add 3.81g of celecoxib, heat to reflux, the solution is clear and transparent, reflux for 2 hours, distill about 5ml Ethanol, cooled to room temperature, let stand for 5 hours, then precipitated co-crystals, filtered, and dried the solid in vacuum at 60-65°C for 6 hours to obtain 6.3 g of tapentadol hydrochloride and celecoxib co-crystals, with a yield of 98.7%.
Embodiment 2
[0039] Example 2 Physical and Chemical Properties Test of Tapentadol Hydrochloride and Celecoxib Cocrystal
[0040] 1. Melting point
[0041] The co-crystals of tapentadol hydrochloride and celecoxib were taken and determined according to the law (Appendix VI C of Part Two of the Chinese Pharmacopoeia 2010 Edition). The melting point of the sample was determined to be 270.4-273.0°C.
[0042] Determined by the same method, the melting point of tapentadol hydrochloride is: 210.5-213.7°C; the melting point of celecoxib is: 161.3-165.1°C
[0043] 2. Hygroscopicity
[0044] Take about 1 g of tapentadol hydrochloride and celecoxib eutectic raw materials, spread it into a flat weighing bottle with constant weight to form a thin layer (thickness is about 5 mm), weigh it accurately, and then place it in air, filled with saturated Place it in a closed container of sodium chloride solution (RH75%) and a closed container filled with saturated potassium nitrate solution (RH92.5%) for 10 da...
Embodiment 3
[0051] Example 3 Structural Characterization of Tapentadol Hydrochloride and Celecoxib Cocrystal
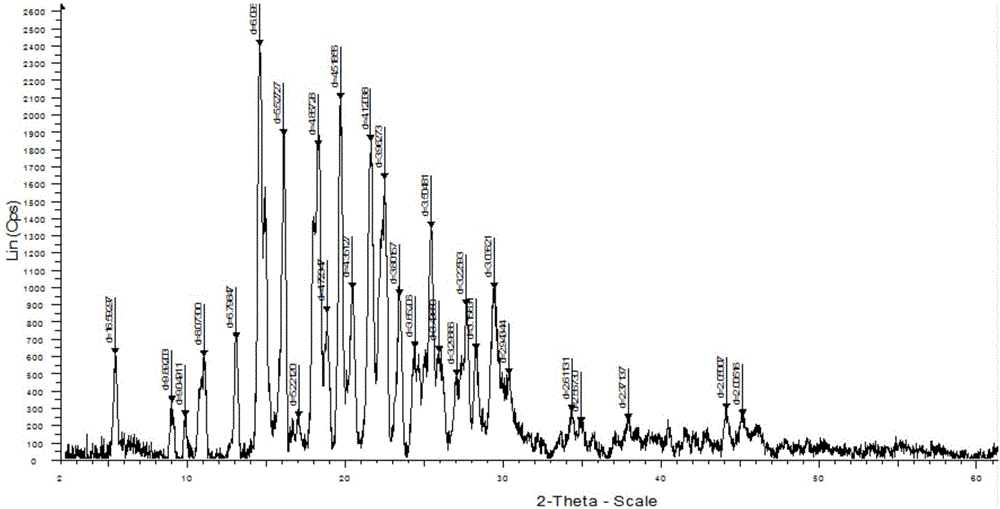
[0052] 1. XRPD characterization
[0053] The XRPD characterization of the co-crystal of tapentadol hydrochloride and celecoxib is listed in the following table:
[0054]
[0055] The entire list of peaks or a subset thereof may be sufficient to characterize the co-crystal. For example, the co-crystal can be characterized by at least 3 peaks selected from the peaks at 16.022, 18.212, 19.631, 21.550, 22.418, 25.393, 29.394 and an XRPD pattern substantially similar to that of Figure 1 .
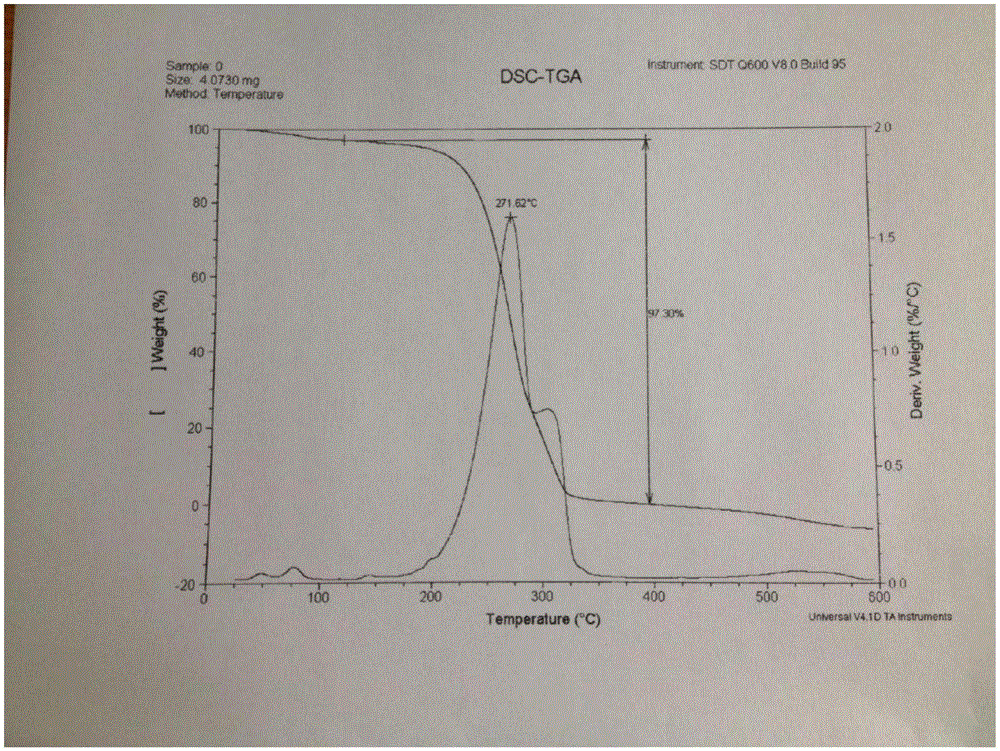
[0056] 2. DSC characterization
[0057] DSC of the co-crystals of tapentadol hydrochloride and celecoxib showed that the co-crystals had a broad endothermic peak around 271°C.
[0058] 3. TG characterization
[0059] The co-crystal TG of tapentadol hydrochloride and celecoxib showed that the co-crystal lost weight significantly at 238°C to 280°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com