Patents
Literature
95 results about "Decreased pain sensitivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hyperalgesia (/ˌhaɪpərælˈdʒiːziə/ or /-siə/; 'hyper' from Greek ὑπέρ (huper, “over”), '-algesia' from Greek algos, ἄλγος (pain)) is an increased sensitivity to pain, which may be caused by damage to nociceptors or peripheral nerves and can cause hypersensitivity to stimulus, stimuli which would normally not be cause for a pain reaction (ex/ eyes or ...
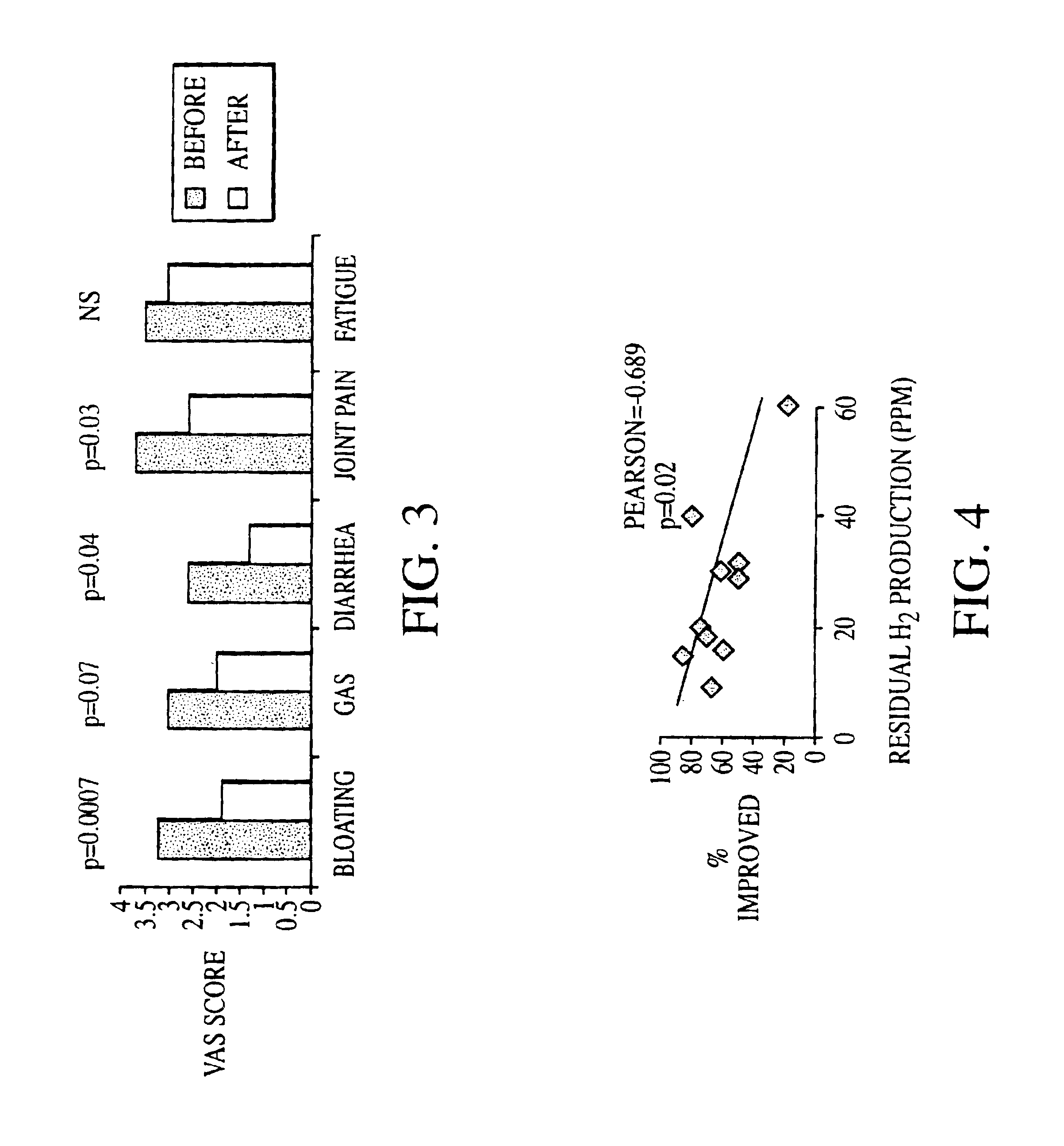
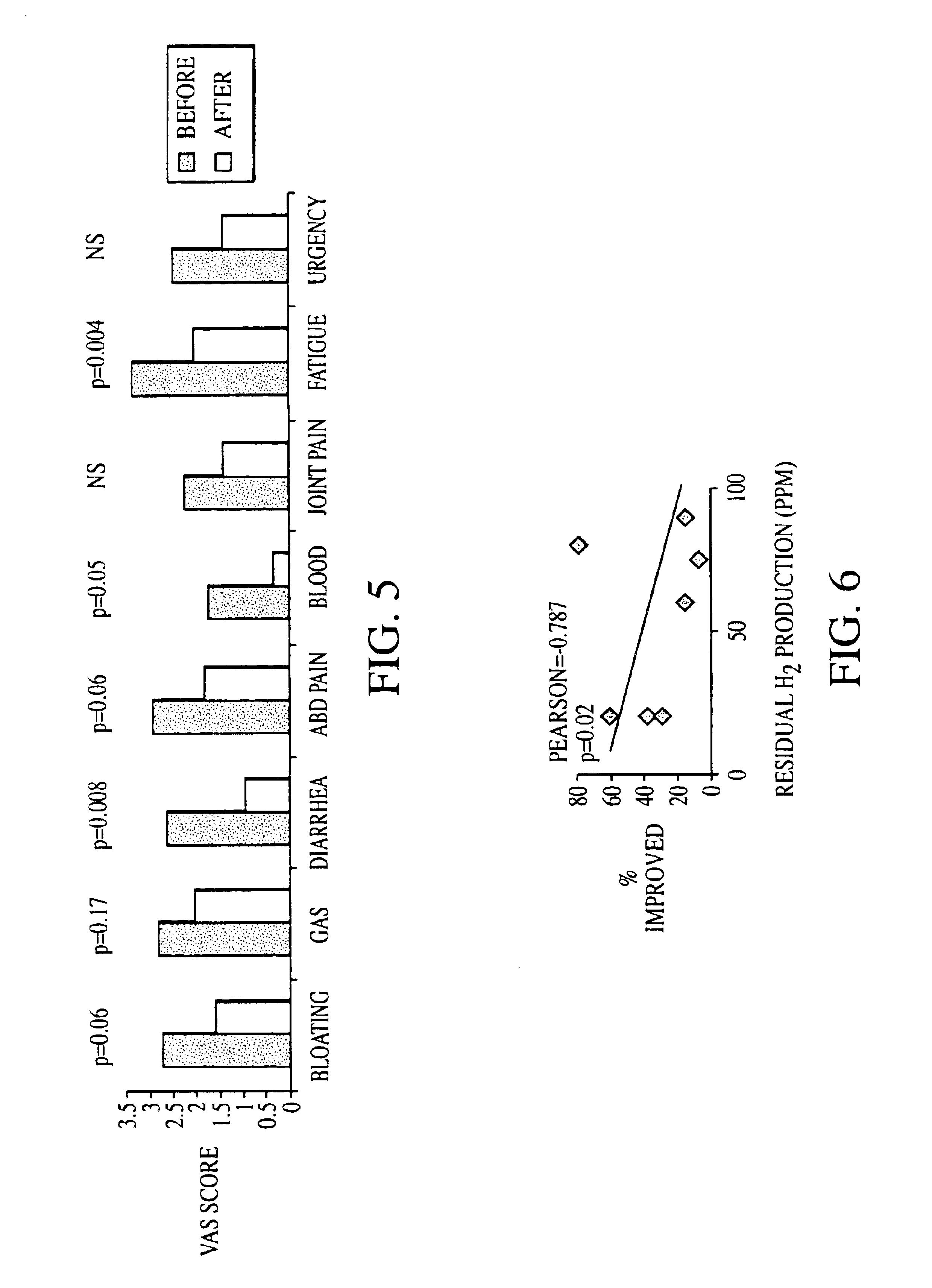
Methods of diagnosing or treating irritable bowel syndrome and other disorders caused by small intestinal bacterial overgrowth
InactiveUS6861053B1Eradicate small intestinal bacterial overgrowthSymptoms improvedAntibacterial agentsOrganic active ingredientsBacteroidesAutoimmune responses
Disclosed is a method of diagnosing irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, depression, attention deficit / hyperactivity disorder, autoimmune diseases, such as multiple sclerosis and systemic lupus erythematosus, or Crohn's disease, which involves detecting the presence of small intestinal bacterial overgrowth (SIBO) in a human subject having at least one symptom associated with a suspected diagnosis of any of those diagnostic categories. Also disclosed is a method of treating these disorders, and other disorders caused by SIBO, that involves at least partially eradicating a SIBO condition in the human subject. The method includes administration of anti-microbial or probiotic agents, or normalizing intestinal motility by employing a prokinetic agent. The method improves symptoms, including hyperalgesia related to SIBO and disorders caused by SIBO. Also disclosed is a kit for the diagnosis or treatment of irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, depression, attention deficit / hyperactivity disorder, autoimmune diseases, or Crohn's disease.
Owner:CEDARS SINAI MEDICAL CENT
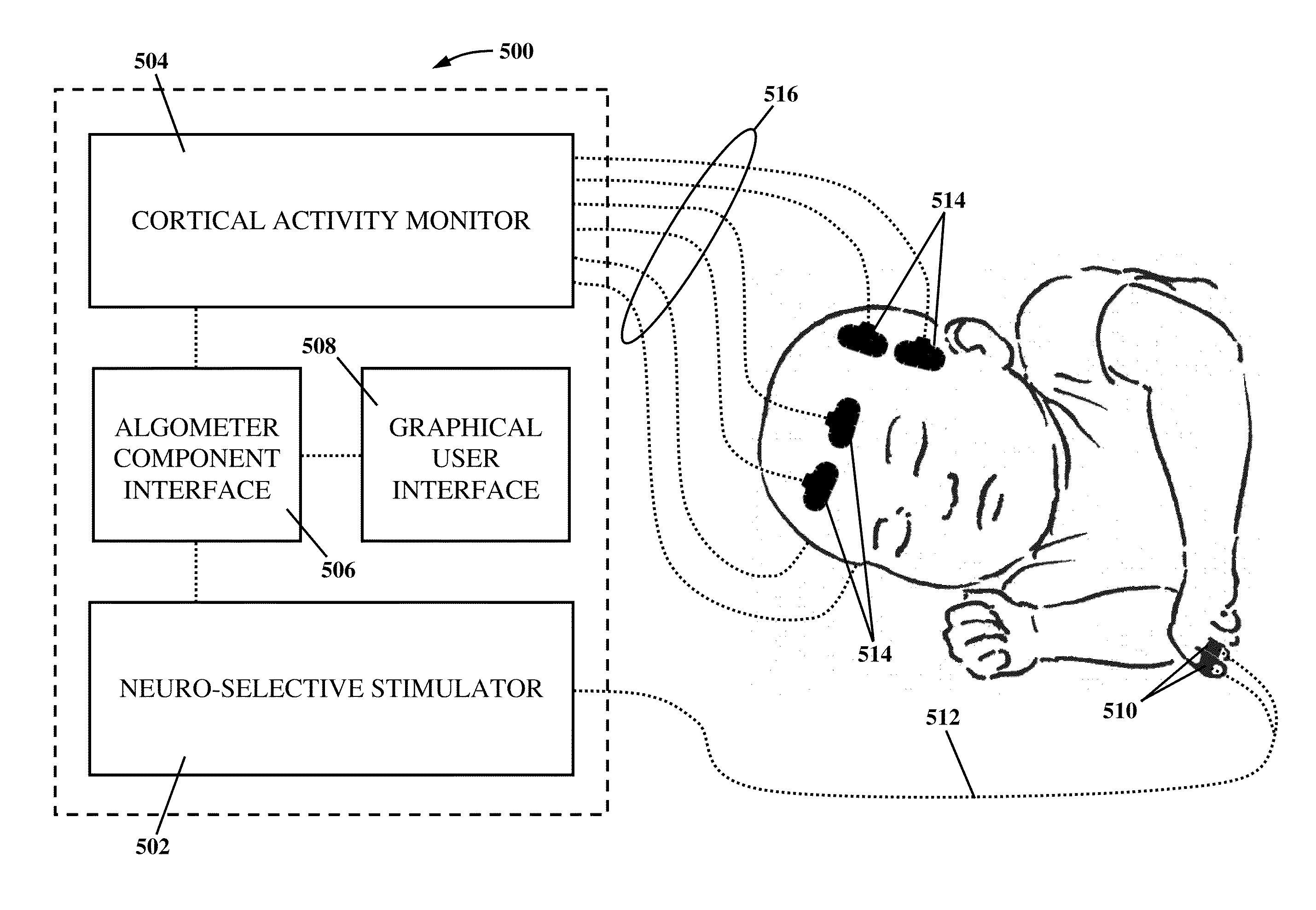
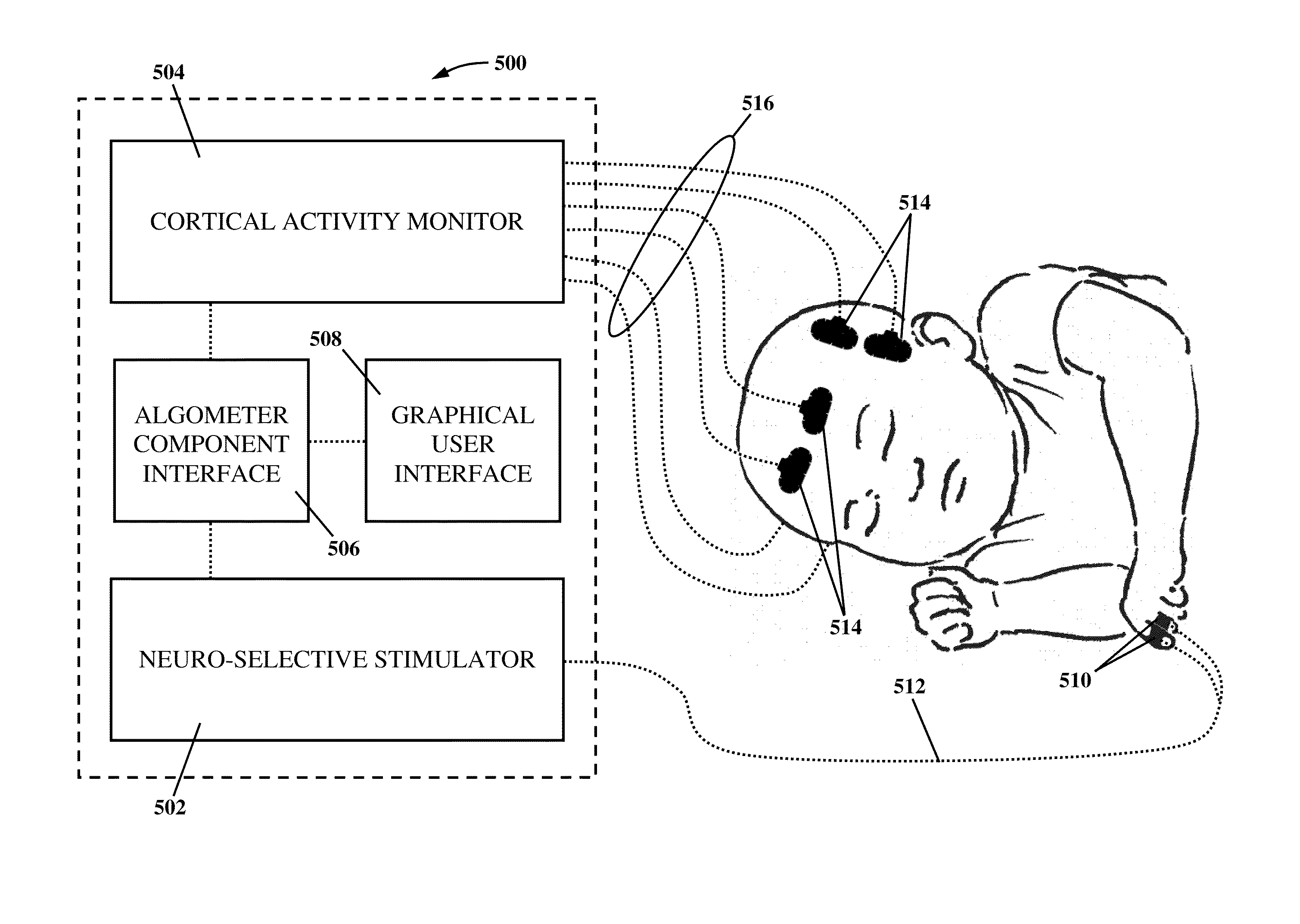
Apparatus and method for human algometry
An apparatus and method for performing human algometry are disclosed. They include a stimulator configured to apply electrical stimulation of variable intensity to an area of a patient's body, a monitoring device configured to measure a. level of cortical activity in one or more regions of the patient's brain, and a microprocessor connected to the stimulator and the monitoring device that is configured to correlate the intensity of the electrical stimulation with the level of activity in the one or more regions of the patient's brain and to determine at least one of a measurement of pain intensity, a measurement of a sensory detection threshold (SDT), a measurement of a drug's analgesic impact, an indication of an onset of tolerance to a drug, an indication of an onset of analgesic-induced hyperalgesia, an indication of conditions of allodynia, a measurement of dose-response characteristics of pain management drugs, and a characterization of a pain condition.
Owner:CHILDRENS NAT MEDICAL CENT
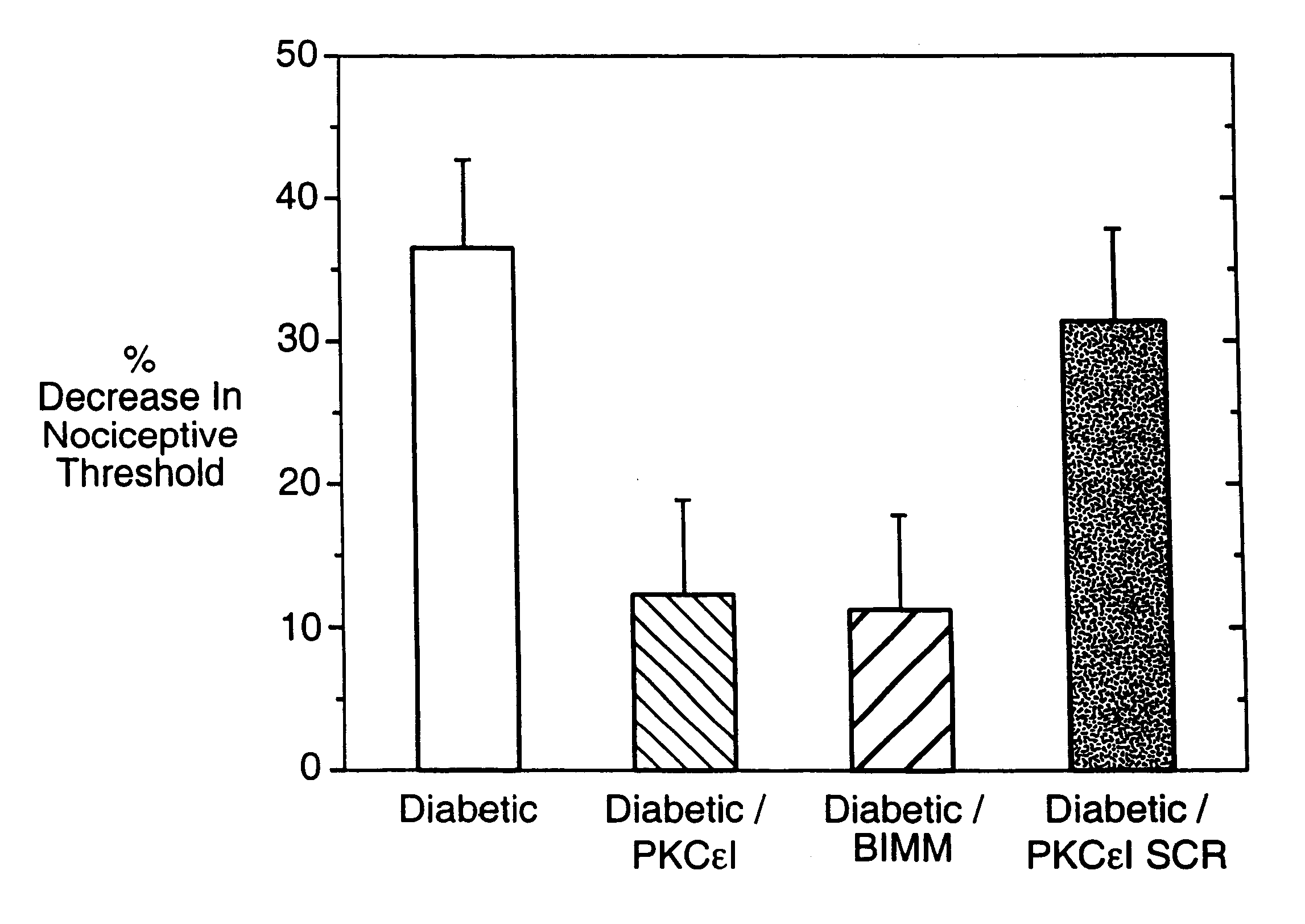
Use of inhibitors of protein kinase C epsilon to treat pain
The role of the epsilon isozyme of protein kinase C ("PKCepsilon") in pain perception, particularly hyperalgesia, methods of lessening pain through administration of inhibitors of PKCepsilon, methods of identifying compounds that modulate pain, and pharmaceutical compositions comprising an inhibitor of PKCepsilon and PKCepsilon-independent analgesic agent are disclosed.
Owner:RGT UNIV OF CALIFORNIA
Method of diagnosing irritable bowel syndrome and other disorders caused by small intestinal bacterial overgrowth
InactiveUS20050008652A1Eradicate small intestinal bacterial overgrowthSymptoms improvedBiocideAntipyreticAutoimmune conditionAutoimmune disease
Disclosed is a method of diagnosing irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, depression, attention deficit / hyperactivity disorder, autoimmune diseases, such as multiple sclerosis and systemic lupus erythematosus, or Crohn's disease, which involves detecting the presence of small intestinal bacterial overgrowth (SIBO) in a human subject having at least one symptom associated with a suspected diagnosis of any of those diagnostic categories. Also disclosed is a method of treating these disorders, and other disorders caused by SIBO, that involves at least partially eradicating a SIBO condition in the human subject. The method includes administration of anti-microbial or probiotic agents, or normalizing intestinal motility by employing a prokinetic agent. The method improves symptoms, including hyperalgesia related to SIBO and disorders caused by SIBO. Also disclosed is a kit for the diagnosis or treatment of irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, depression, attention deficit / hyperactivity disorder, autoimmune diseases, or Crohn's disease.
Owner:CEDARS SINAI MEDICAL CENT
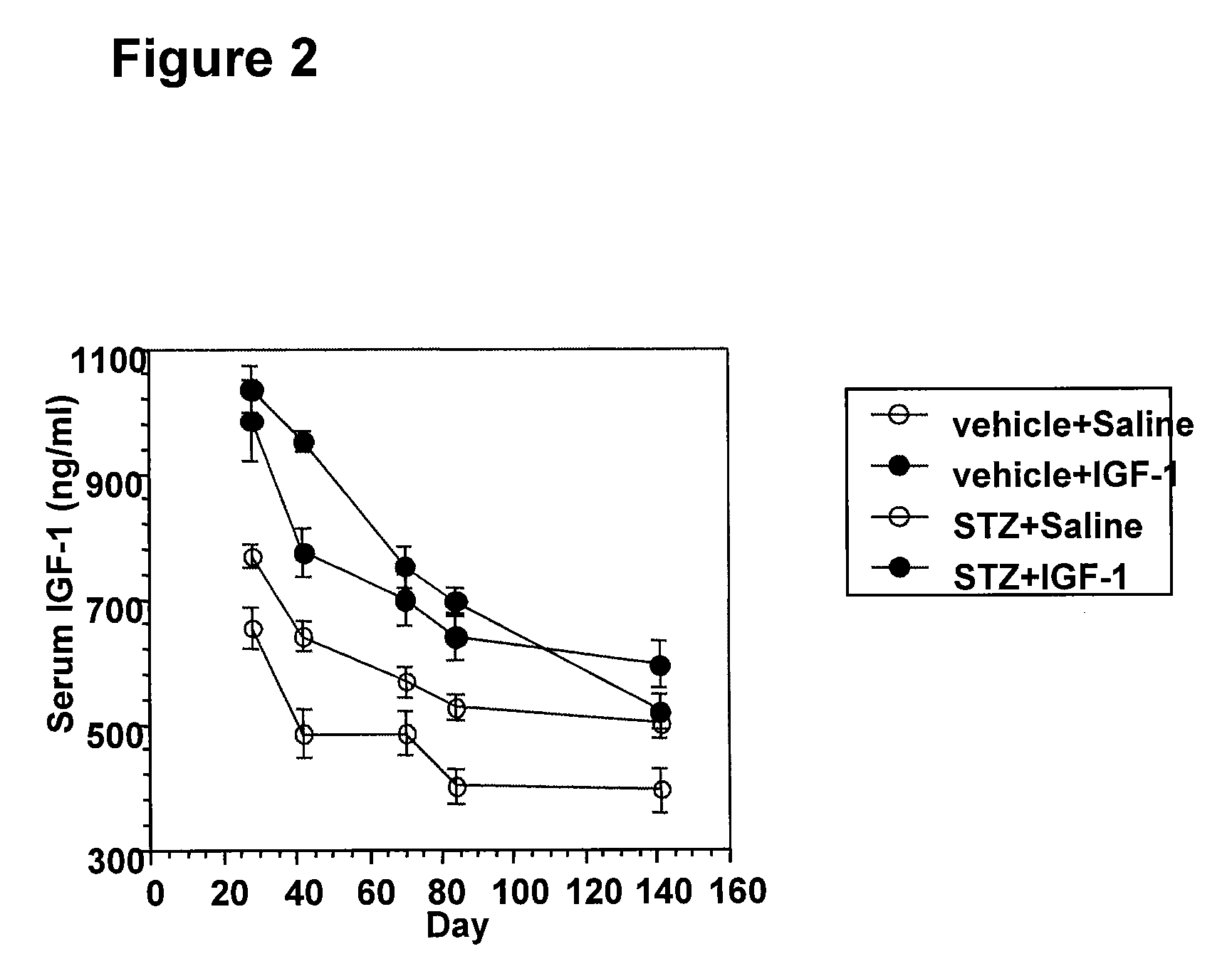
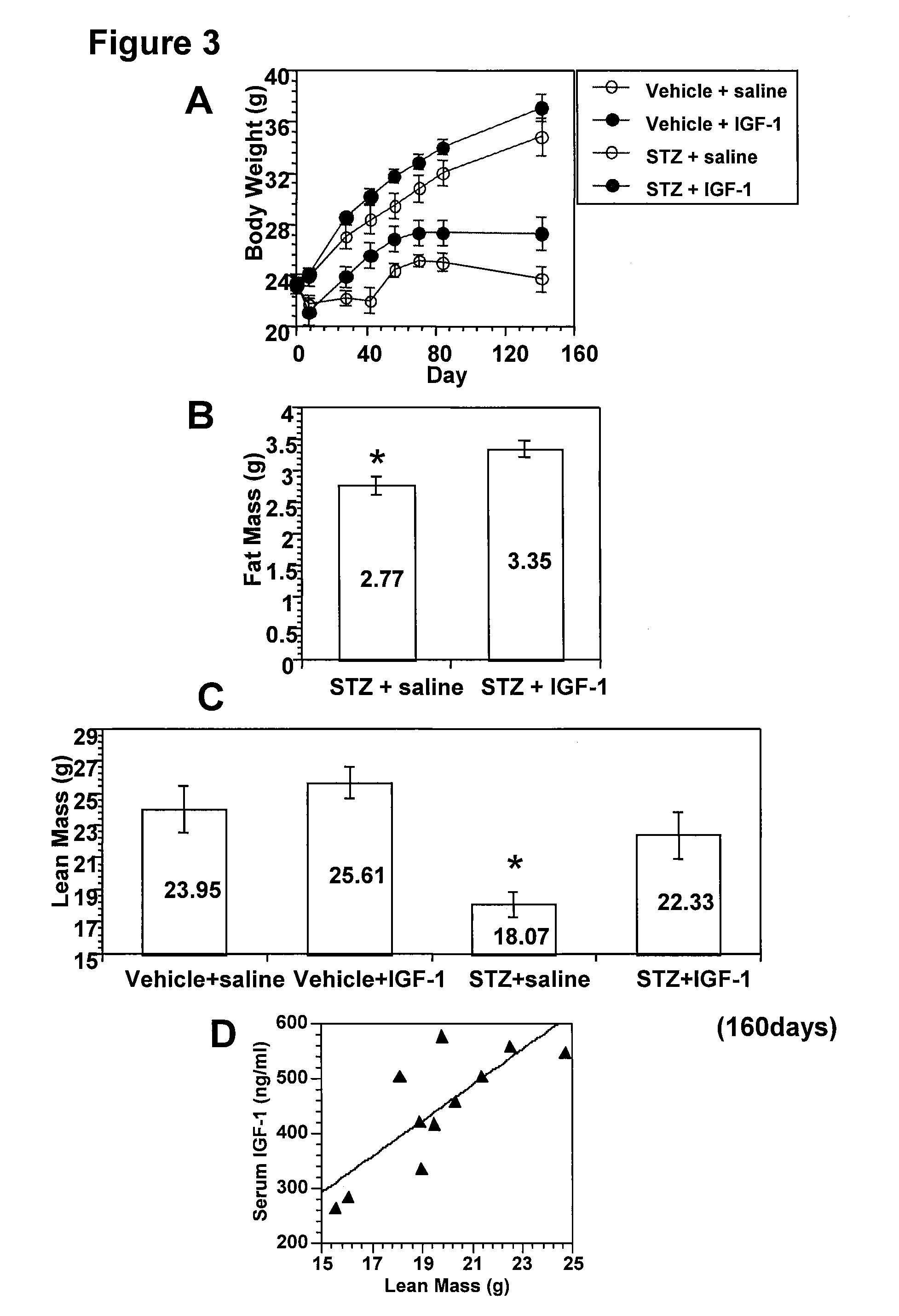
Systemic insulin-like growth factor-1 therapy reduces diabetic peripheral neuropathy and improves renal function in diabetic nephropathy
InactiveUS20100216709A1Prevents subsequent hyposensitivityEasy maintenanceOrganic active ingredientsNervous disorderInsulin-like growth factorHyperglycemic disorder
The present invention provides methods of treatment of patients suffering from the complications of blood sugar disorders: diabetic peripheral neuropathy and diabetic nephropathy by administration of IGF-1 via protein therapy or gene therapy. It relates to methods of treating an individual having a diabetic disorder or a hyperglycemic disorder, comprising administering to the individual an effective amount of a DNA vector expressing IGF-1Eb or IGF-1Ec in vivo or an effective amount of at the IGF-1Eb or IGF-1Ec protein in the early hyperalgesia stage or in patients that have advanced to the hyposensitivity stage. Treatment at the early hyperalgesia stage prevents subsequent hyposensitivity with increases or maintenance of sensory nerve function. IGF-1Eb or IGF-1Ec treatment also increases muscle mass and improves overall mobility, which indicates a treatment-related improvement in motor function. Treatment with IGF-1Eb or IGF-1Ec at the hyposensitivity stage reverses hyposensitivity and improves muscle mass and overall health. Systemic IGF-1 provides a therapeutic modality for treating hyposensitivity associated with DPN. In addition, IGF-1Eb or IGF-1Ec provides a therapeutic modality for treating diabetic nephropathy. IGF-1Eb or IGF-1Ec improves renal function as evidenced by a modulation in serum albumin concentration and a reduction in urine volume and protein levels. IGF-1Eb or IGF-1Ec also reduces diabetic glomerulosclerosis.
Owner:GENZYME CORP
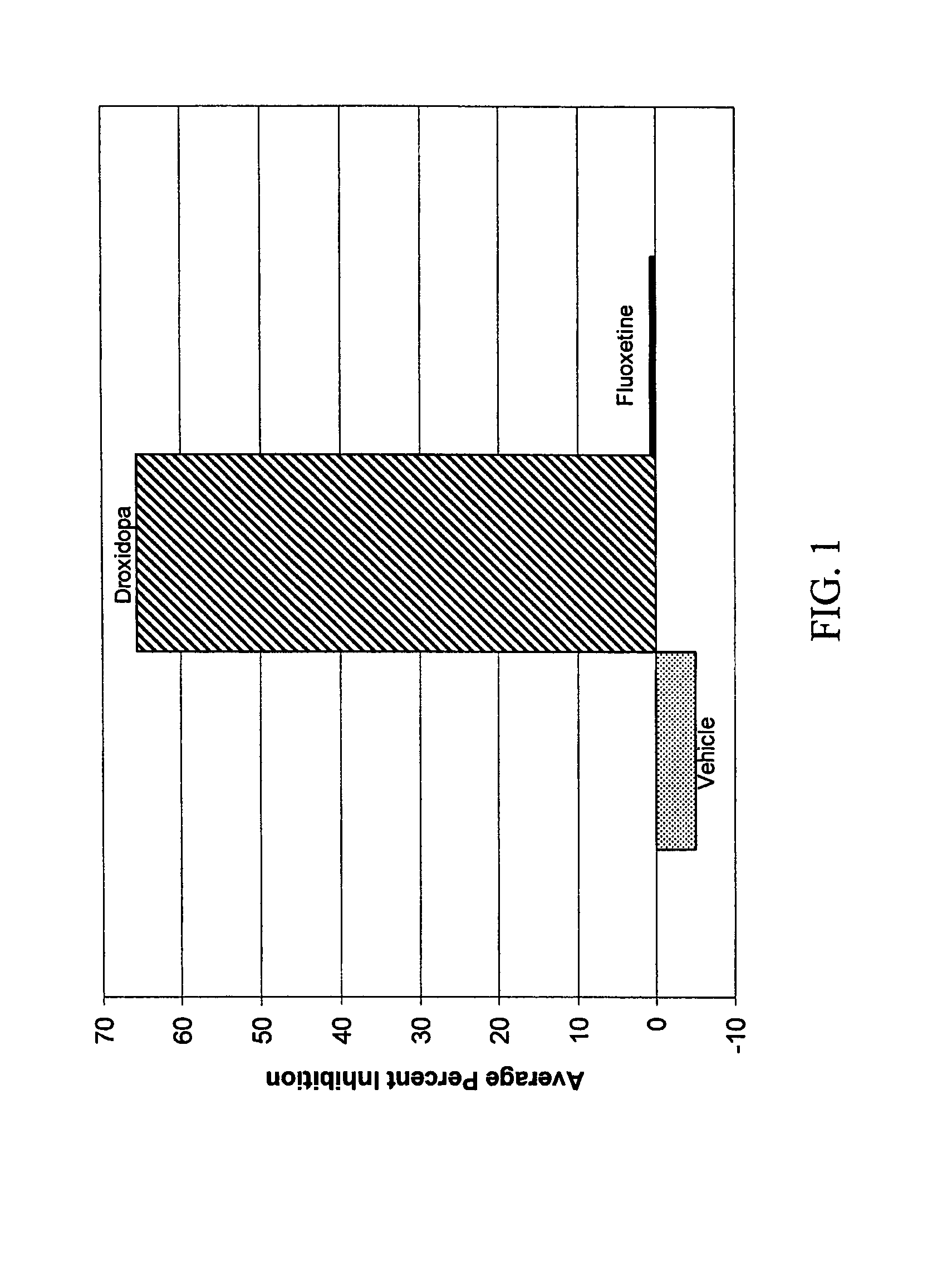
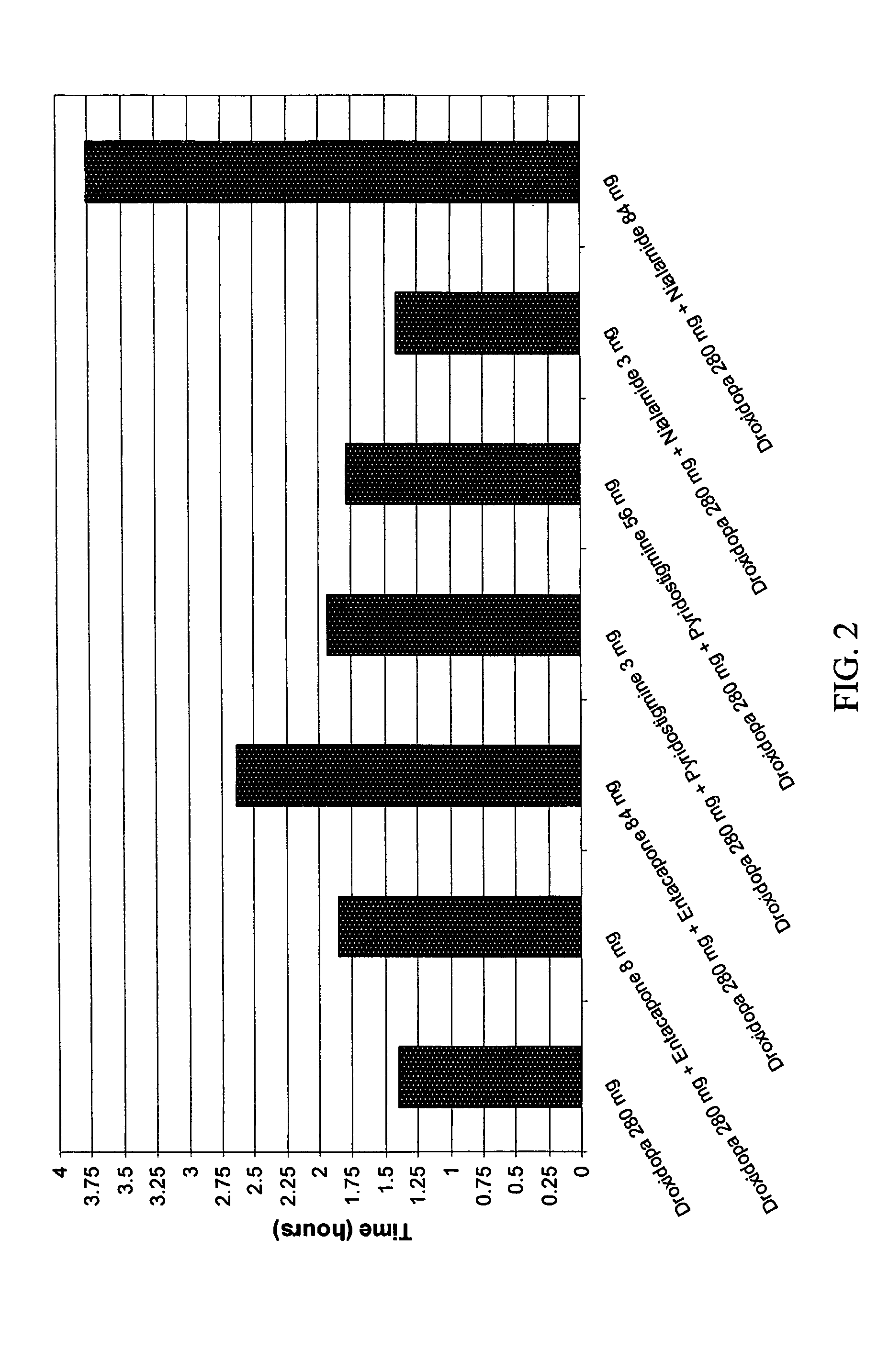
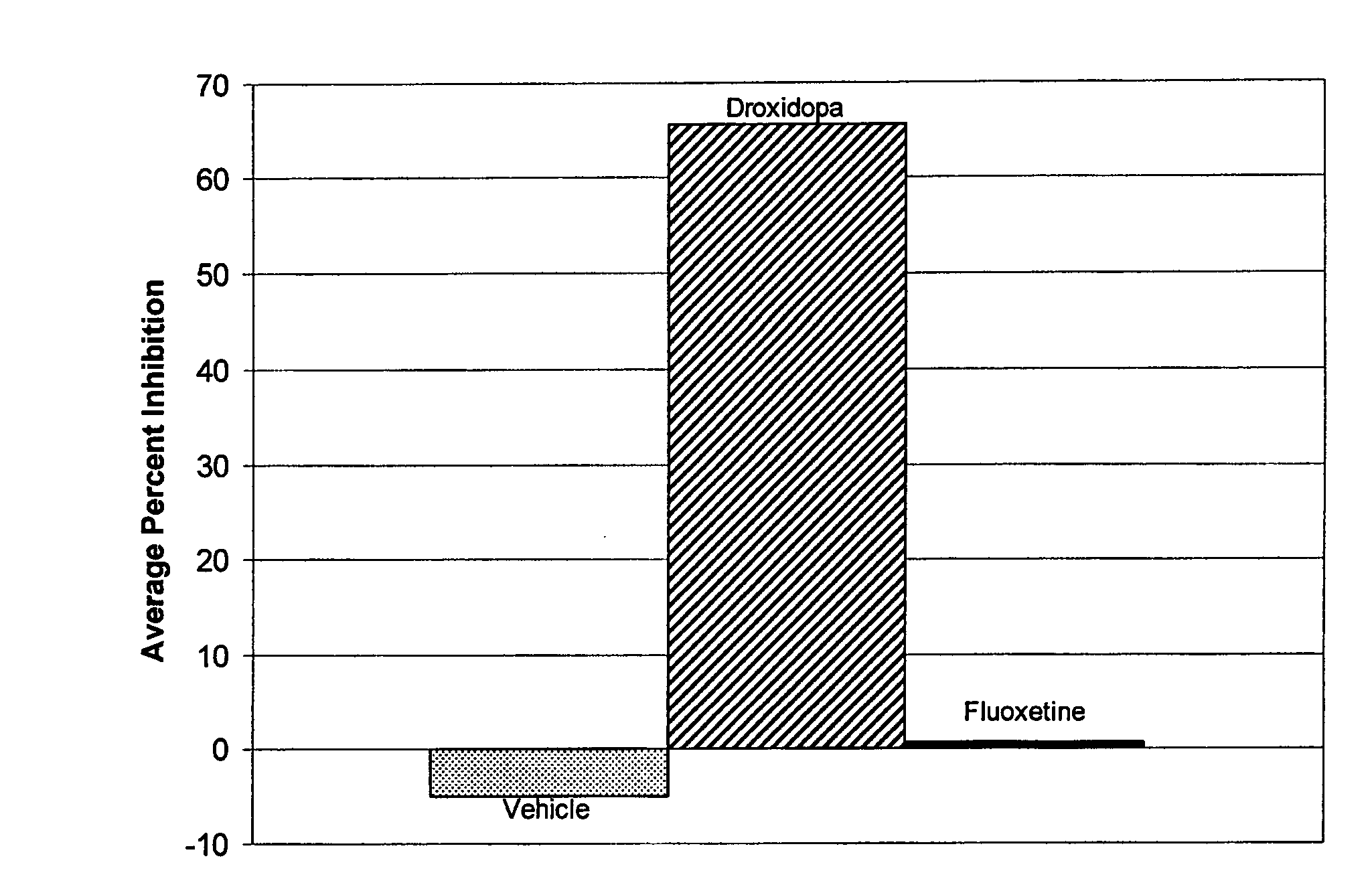
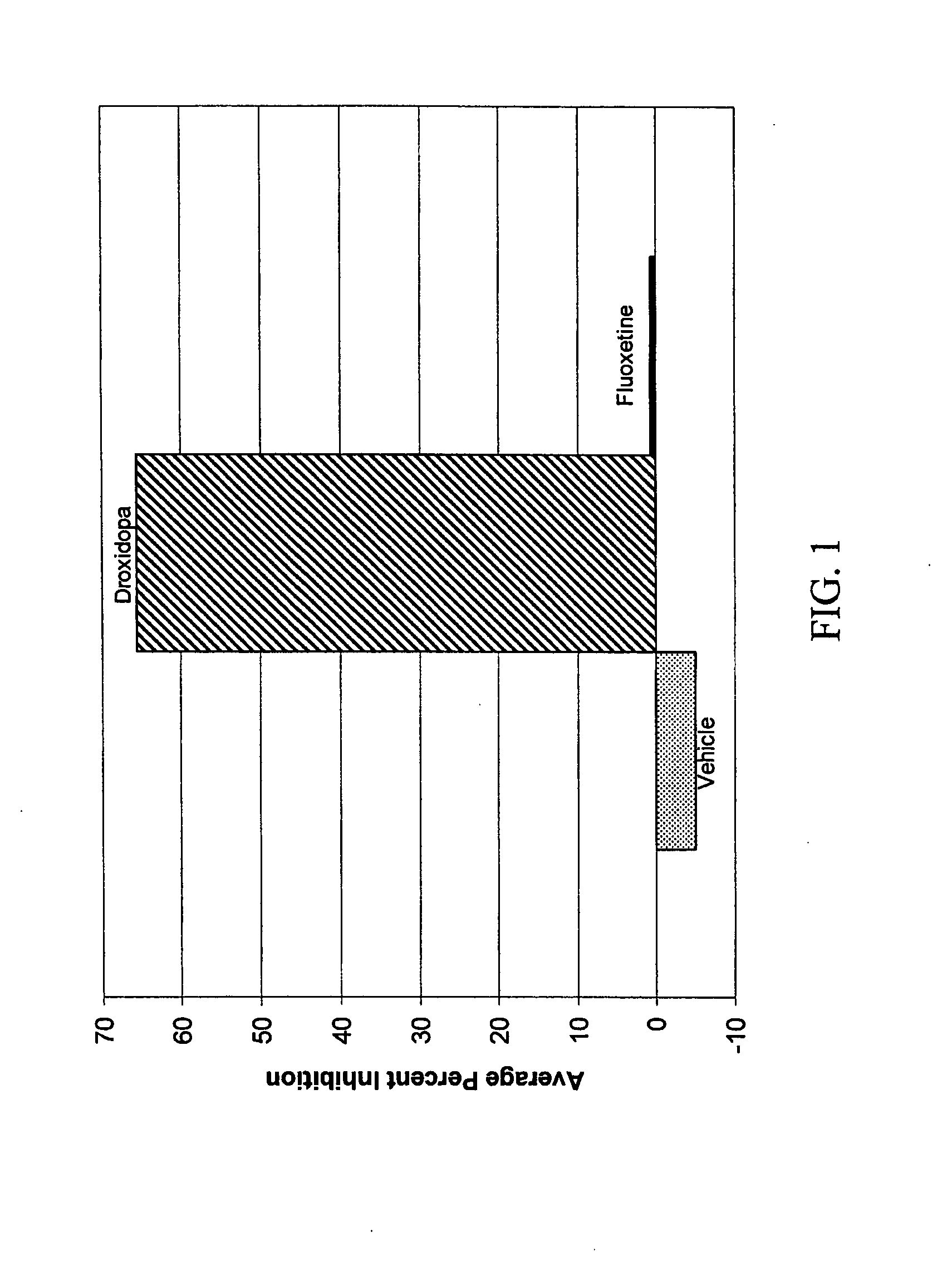
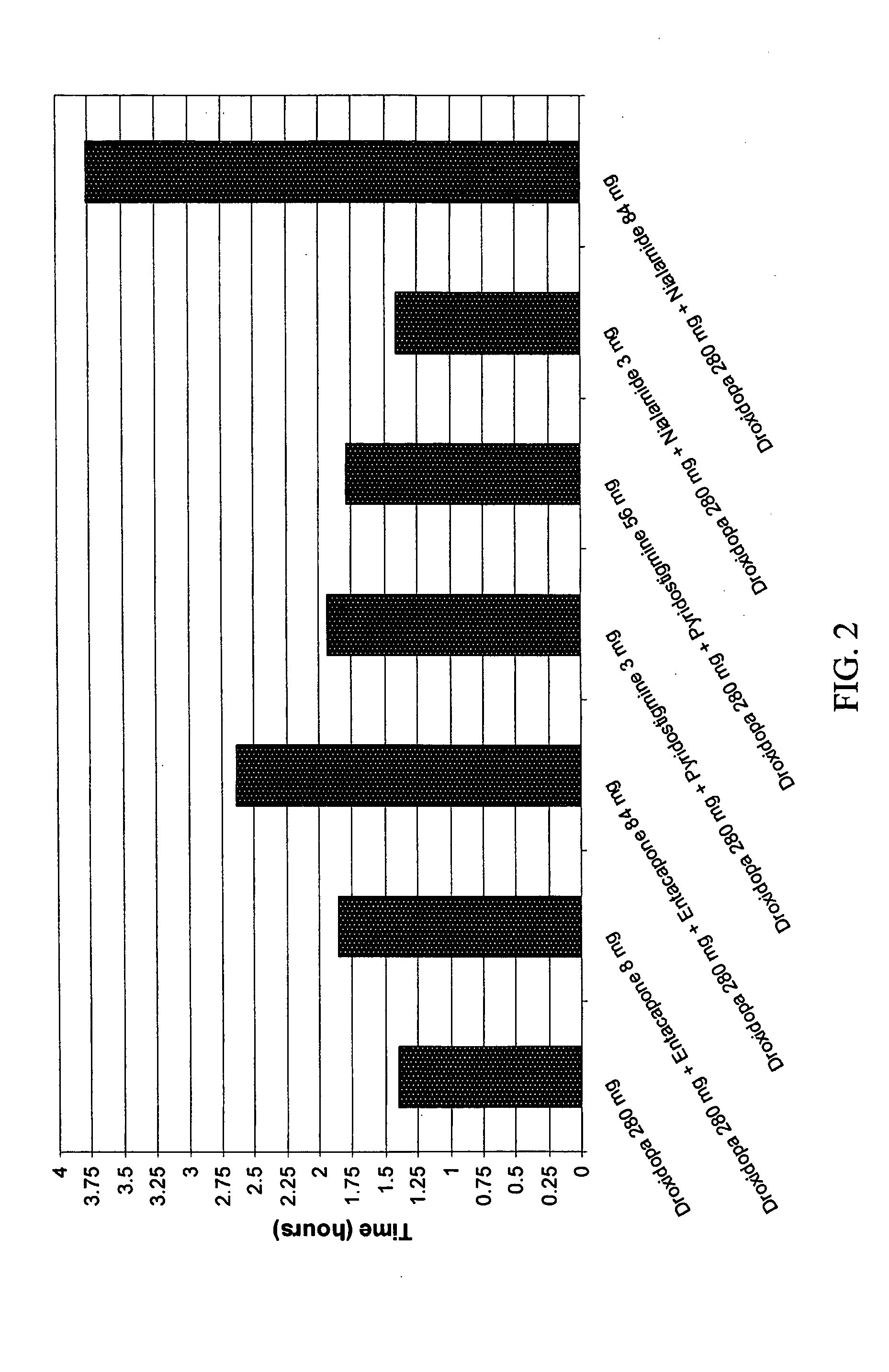
Droxidopa and pharmaceutical composition thereof for the treatment of fibromyalgia
ActiveUS8008285B2Increase choiceReduce chronic painBiocideNervous disorderChronic Widespread PainActive agent
The present invention provides methods of treating fibromyalgia or other diseases or conditions causing widespread pain and / or fatigue. In particular, the invention provides pharmaceutical compositions comprising droxidopa alone, or in combination with one or more further active agents, that can be used in the inventive methods. The methods of treatment can comprise treating, preventing, reducing, or eliminating a variety of symptoms recognized as indicative of fibromyalgia, such as chronic pain, allodynia, hyperalgesia, fatigue, sleep disturbance, and depression.
Owner:CHELSEA THERAPEUTICS
Compositions and methods for enhancing analgesic potency of covalently bound-compounds, attenuating its adverse side effects, and preventing their abuse
InactiveUS20100144645A1Lower potentialAmenable to synthesizing conjugatesBiocideNervous disorderChemical MoietyOpioid antagonist
The invention generally relates to compositions and methods with covalently bound compounds, such as controlled substances covalently attached to a chemical moiety, and opioid antagonists or covalently bound opioid antagonists to enhance analgesic potency and / or attenuate one or more adverse effects of covalently bound compounds, including adverse side effect(s) in humans such as nausea, vomiting, dizziness, headache, sedation (somnolence), physical dependence or pruritis. This invention relates to compositions and methods for selectively enhancing the analgesic potency of a covalently bound compound and simultaneously attenuating anti-analgesia, hyperalgesia, hyperexcitability, physical dependence and / or tolerance effects associated with the administration of a covalently bound compound. The methods of the invention comprise administering to a subject an analgesic or sub-analgesic amount of a covalently bound compound and an amount of excitatory opioid receptor antagonist such as naltrexone or nalmefene effective to enhance the analgesic potency of a covalently bound compound and attenuate the anti-analgesia, hyperalgesia, hyperexcitability, physical dependence and / or tolerance effects of covalently bound compound. The invention also relates to the addition of covalently-bound opioid antagonists to the compositions containing covalently bound compounds such that if the compositions are subjected to manipulation by illicit chemists, the opioid antagonist is released effectively reducing or eliminating the euphoric effect of the covalently bound compounds.
Owner:SHIRE PLC
Compositions and methods for treating pain
ActiveUS20060280718A1Reduce severityReduce thrombosisBiocideNervous disorderNervous systemPost injury
In the present invention, Applicants demonstrate the effect of a biomembrane sealing agent on the development of chronic pain following tissue injury as well as acute pain in a model of acute inflammation. Applicants demonstrate the ability of this class of agents referred to as “biomembrane sealing agents” to reduce the severity of hyperalgesia and allodynia following mechanical insult to the nervous system as well as their ability to reduce acute pain in a model of acute inflammation. Applicant describes the use of injectable or depot formulations of biomembrane sealing agent(s) for prophylactic treatment such as they could be administered after the insult (i.e. post-injury or post-surgery) but before the onset of acute or chronic pain. Alternatively, biomembrane sealing agents could be used to reduce the severity of acute or chronic pain after onset.
Owner:WARSAW ORTHOPEDIC INC
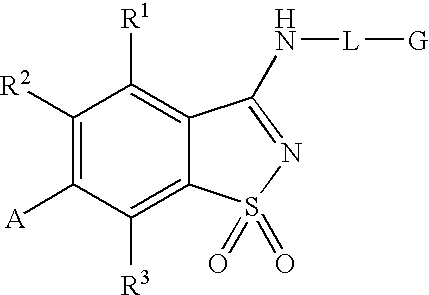
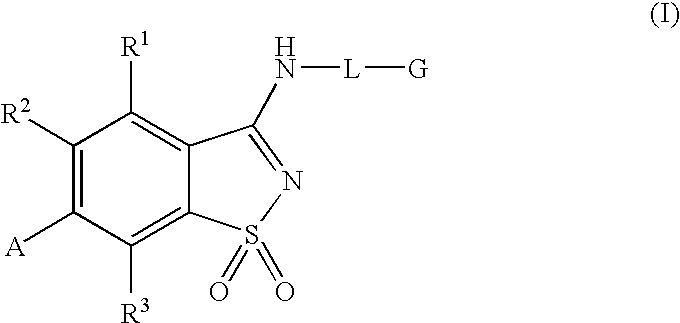
Antagonists to the vanilloid receptor subtype 1 (VR1) and uses thereof
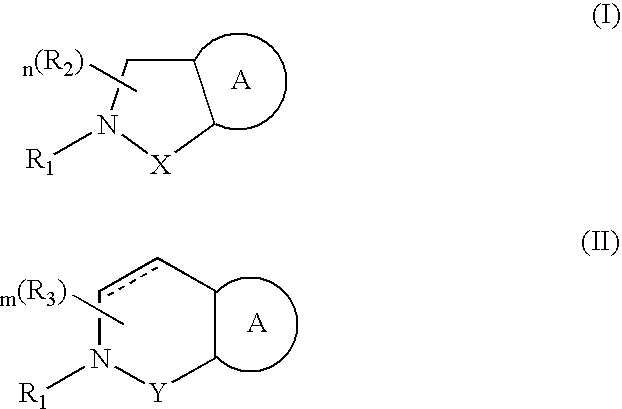
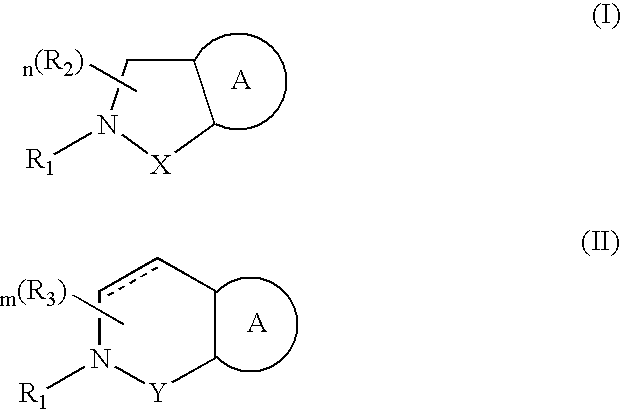
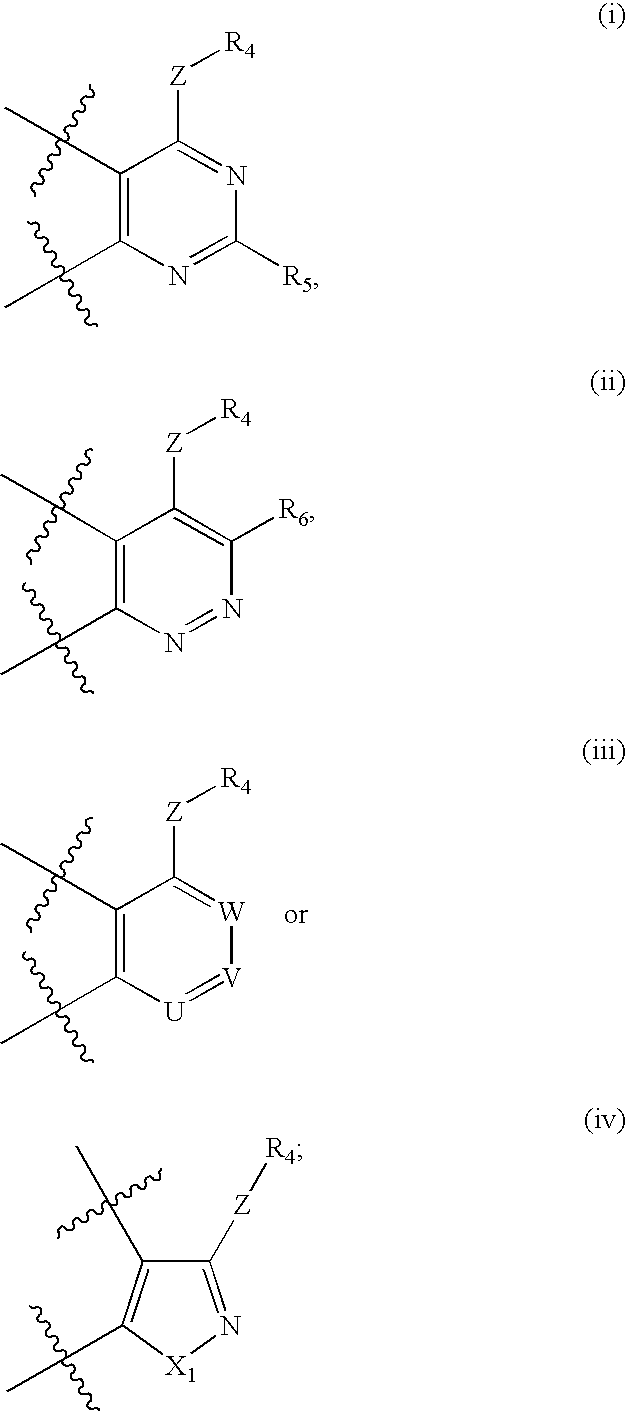
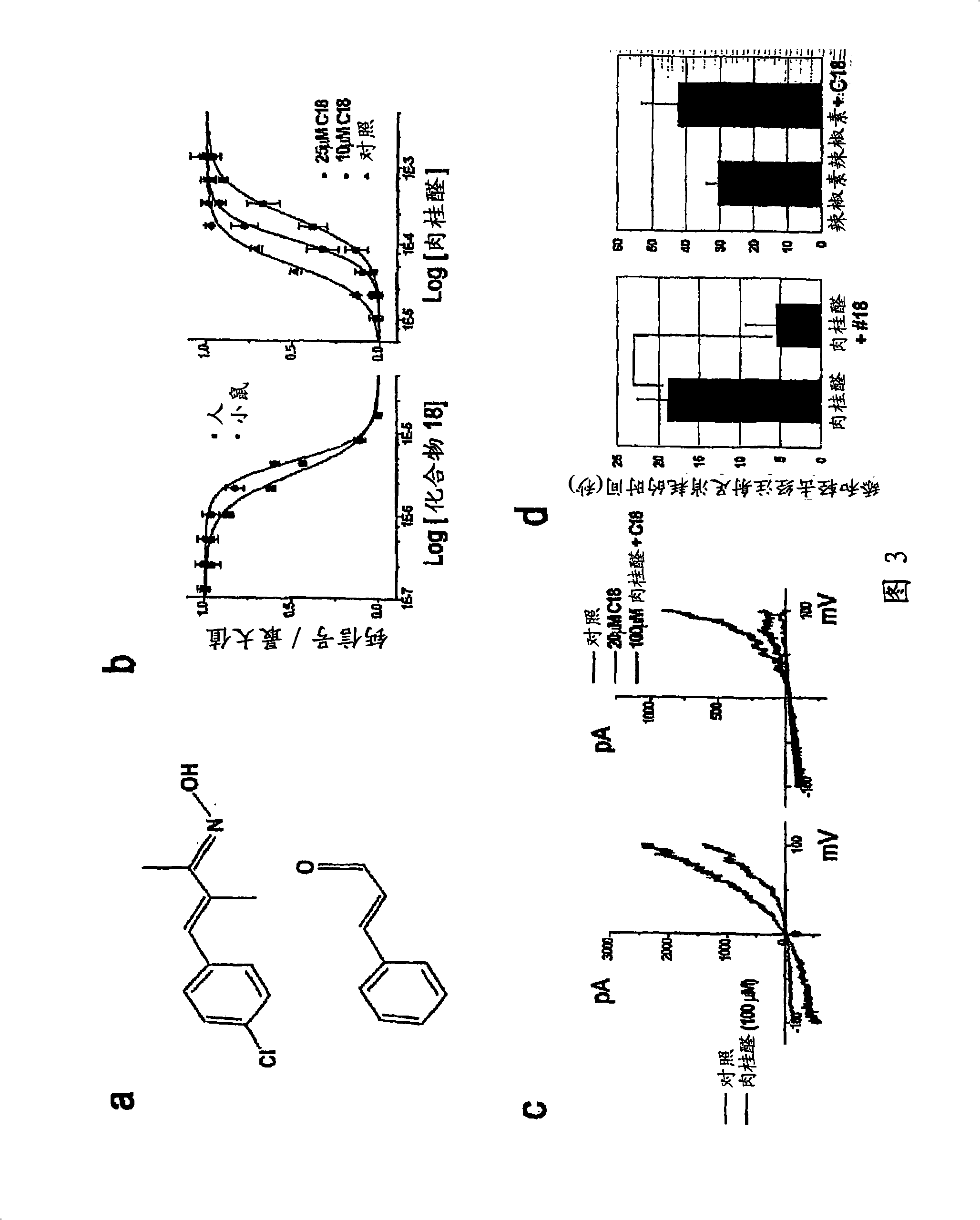
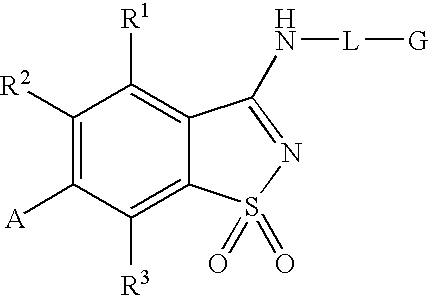
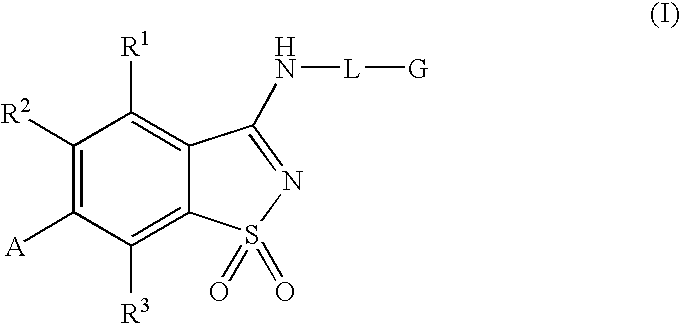
Compounds having formula (I) or formula (II) or a pharmaceutically acceptable salt, prodrug, or salt of a prodrug thereof, wherein A, N, X, Y, R1, R2 and R3 are as defined in the specification. These compounds are particularly useful in the treatment of pain, inflammatory hyperalgesia, and urinary dysfunctions, such as bladder overactivity and urinary incontinence.
Owner:ABBOTT LAB INC
Droxidopa and pharmaceutical composition thereof for the treatment of fibromyalgia
The present invention provides methods of treating fibromyalgia or other diseases or conditions causing widespread pain and / or fatigue. In particular, the invention provides pharmaceutical compositions comprising droxidopa alone, or in combination with one or more further active agents, that can be used in the inventive methods. The methods of treatment can comprise treating, preventing, reducing, or eliminating a variety of symptoms recognized as indicative of fibromyalgia, such as chronic pain, allodynia, hyperalgesia, fatigue, sleep disturbance, and depression.
Owner:CHELSEA THERAPEUTICS
Antagonists to the vanilloid receptor subtype 1 (VR1) and uses thereof
Compounds having formula (I) or a pharmaceutically acceptable salt, prodrug, or salt of a prodrug thereof, wherein L, A, G, R1, R2 and R3 are as defined herein. These compounds are particularly useful in the treatment of pain, inflammatory hyperalgesia, and urinary dysfunctions, such as bladder overactivity and urinary incontinence.
Owner:ABBVIE INC
Use of inhibitors of protein kinase C epsilon to treat pain
The role of the epsi isozyme of protein kinase C ("PKCepsi") in pain perception, particularly hyperalgesia, methods of lessening pain through administration of inhibitors of PKCepsi, methods of identifying compounds that modulate pain, and pharmaceutical compositions comprising an inhibitor of PKCepsi and PKCepsi-independent analgesic agent are disclosed.
Owner:RGT UNIV OF CALIFORNIA
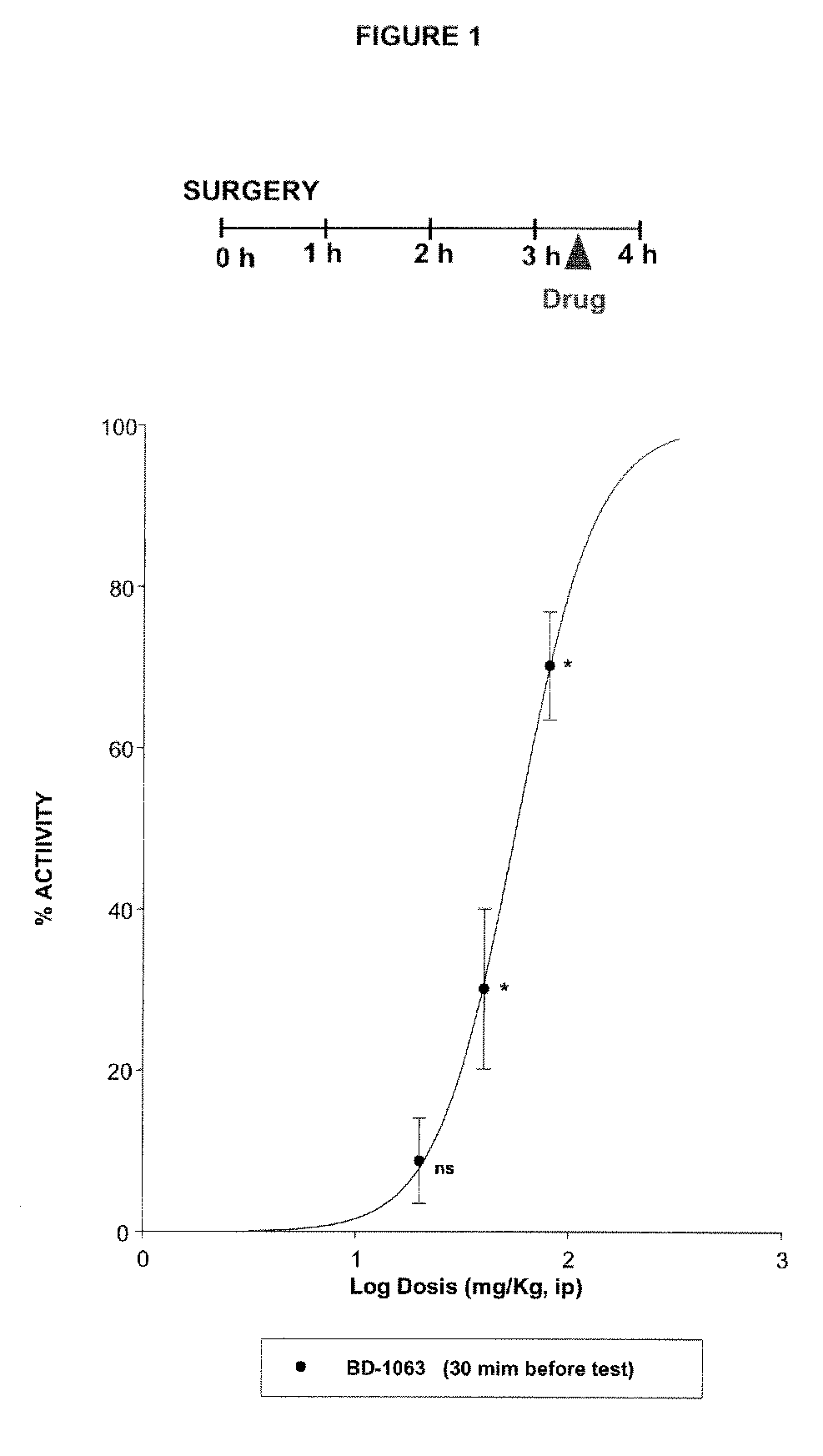
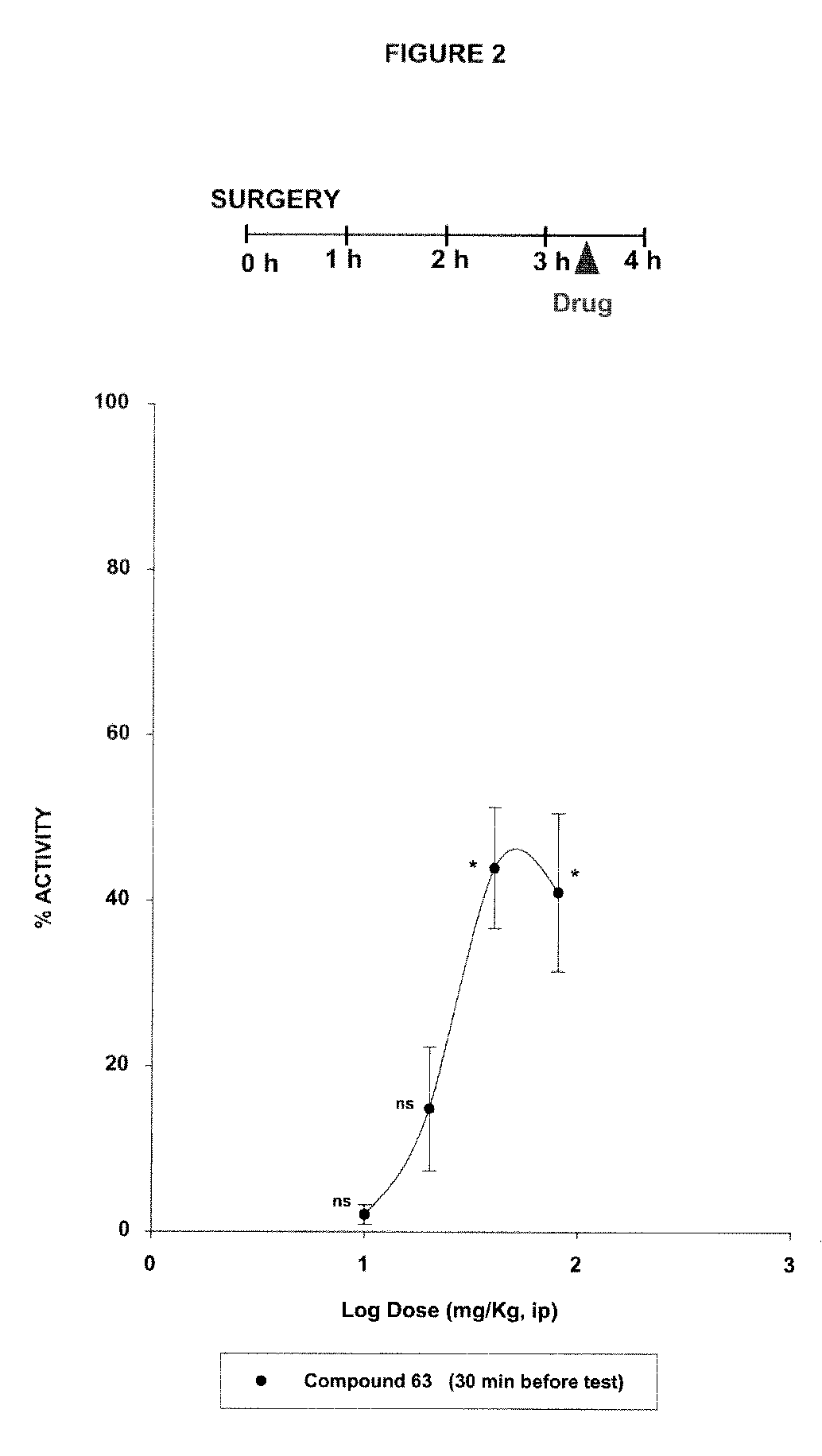
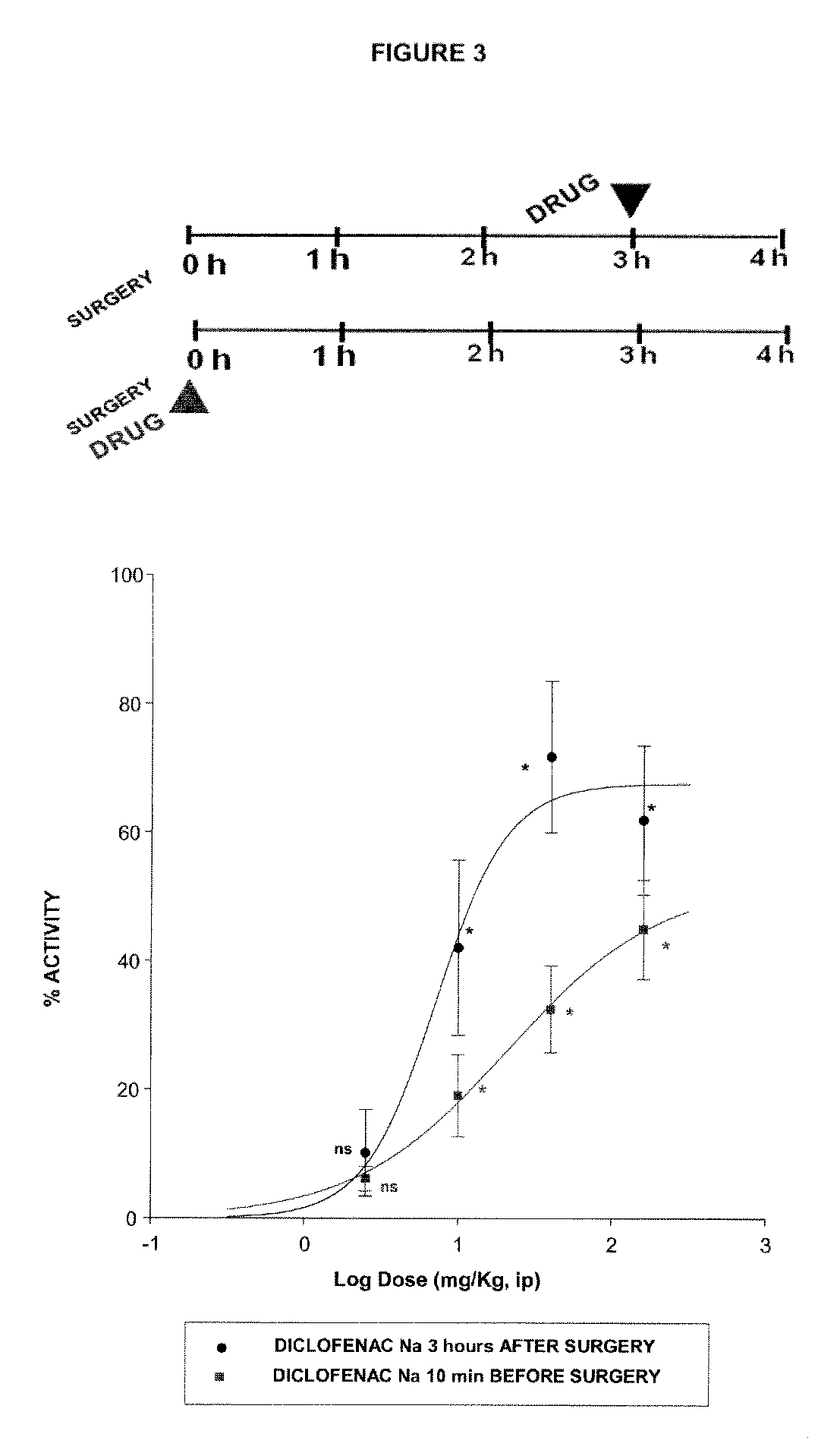
Sigma ligands for use in the prevention and/or treatment of post-operative pain
The invention refers to the use of a sigma ligand, particularly a sigma ligand of formulae (I), (II) or (III) to prevent and / or treat acute and chronic pain developed as a consequence of surgery, especially superficial and / or deep pain secondary to surgical tissue injury, and peripheral neuropathic pain, neuralgia, allodynia, causalgia, hyperalgesia, hyperesthesia, hyperpathia, neuritis or neuropathy secondary to surgical procedure.
Owner:LAB DEL DR ESTEVE SA
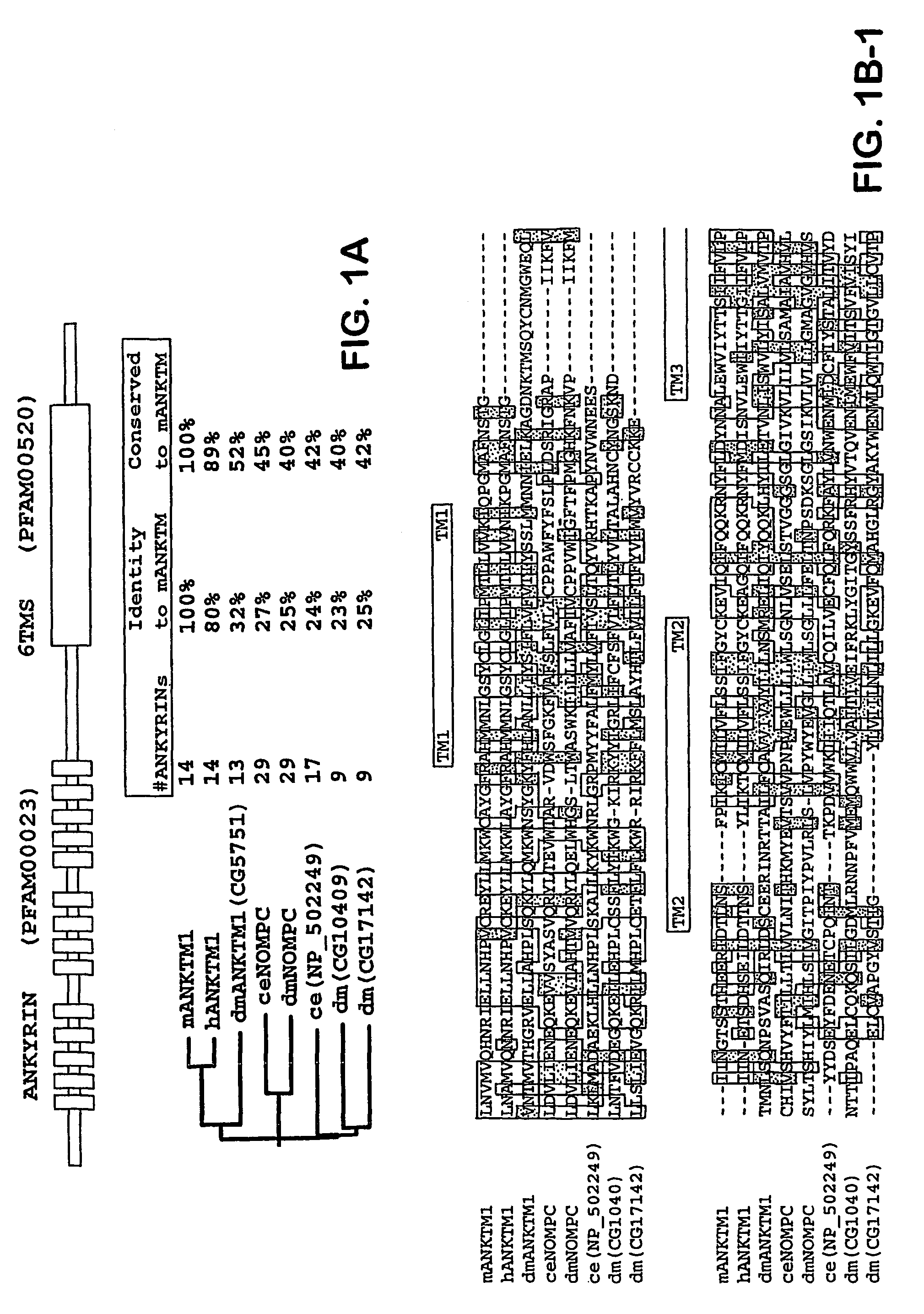
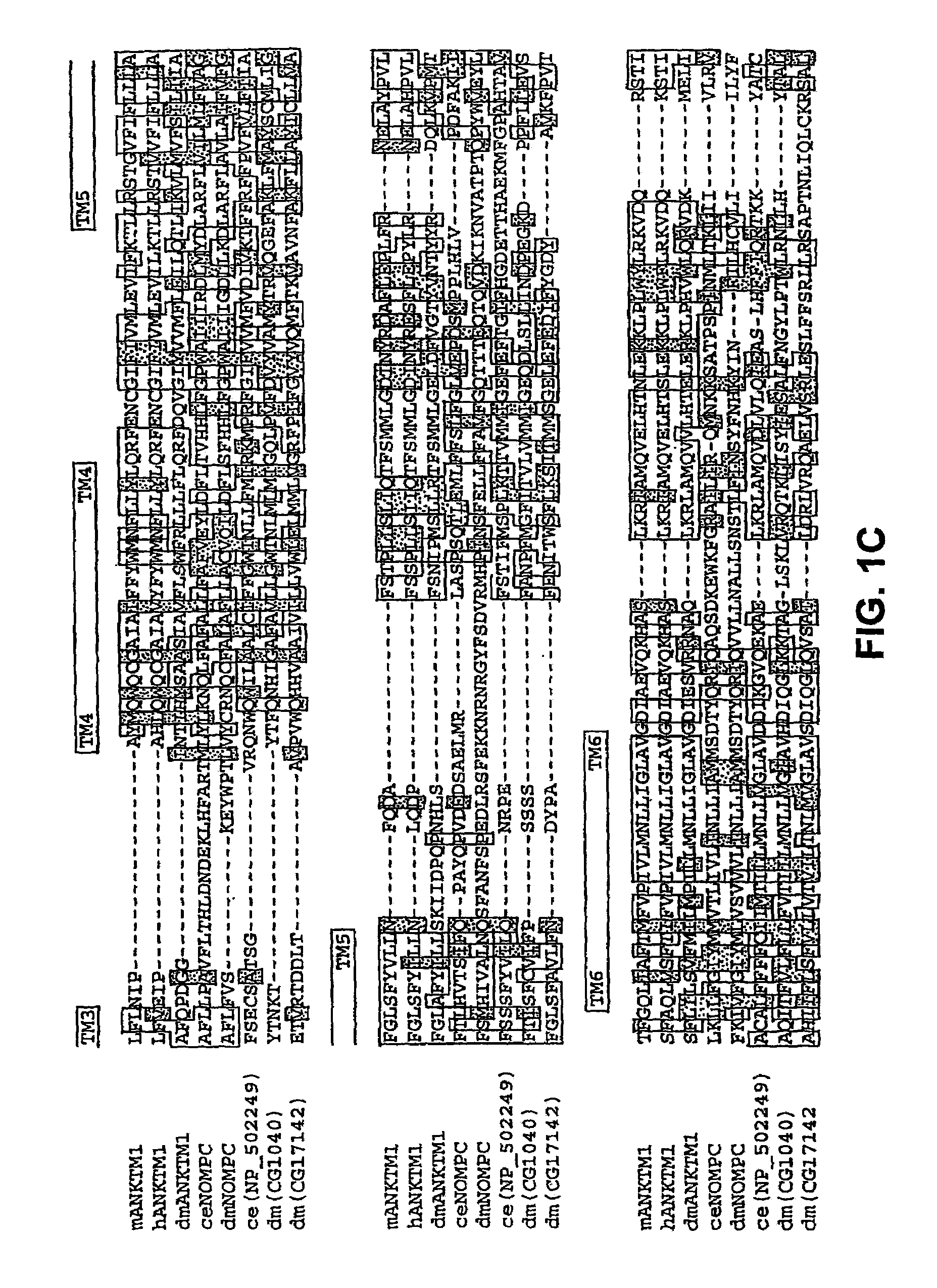
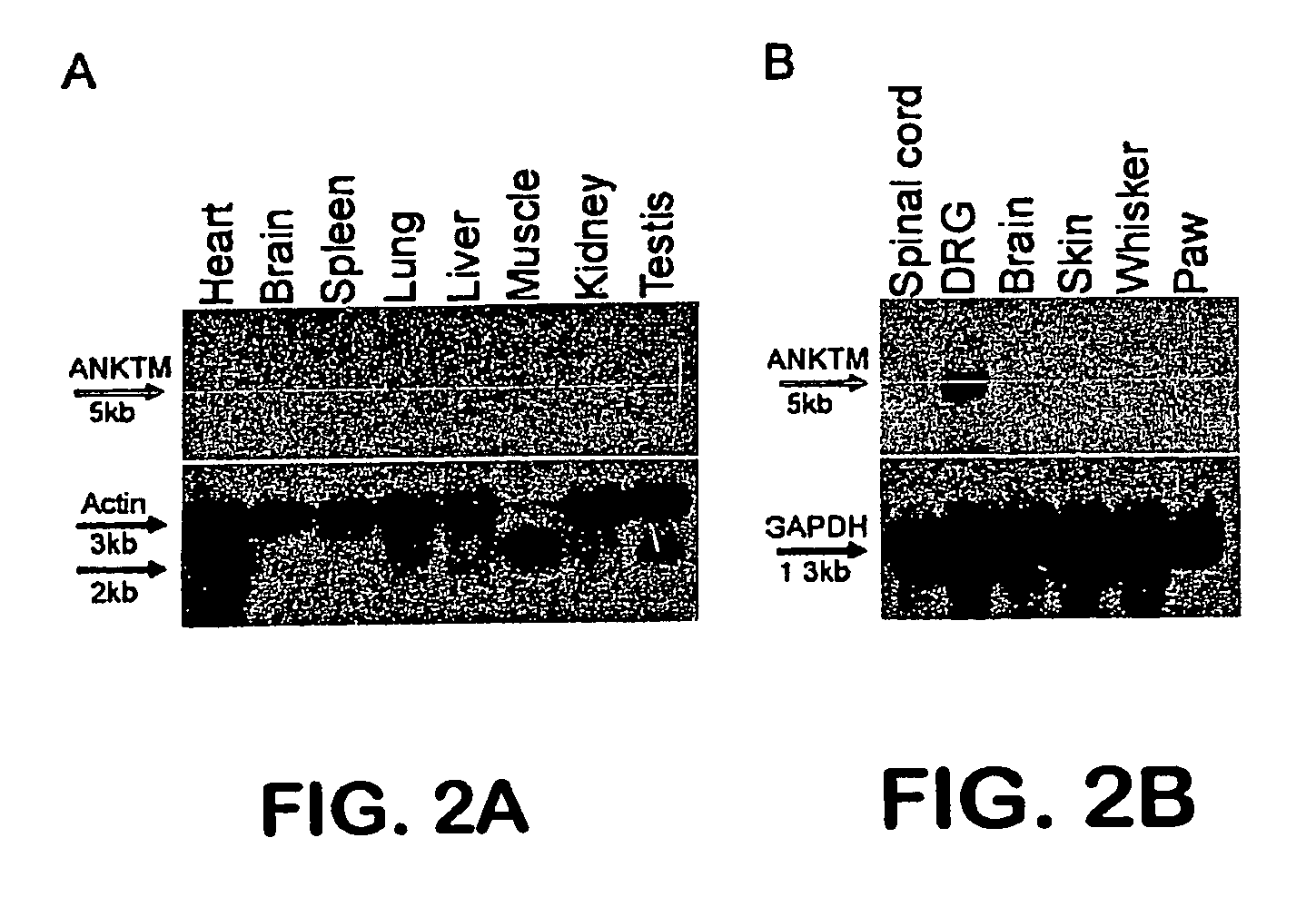
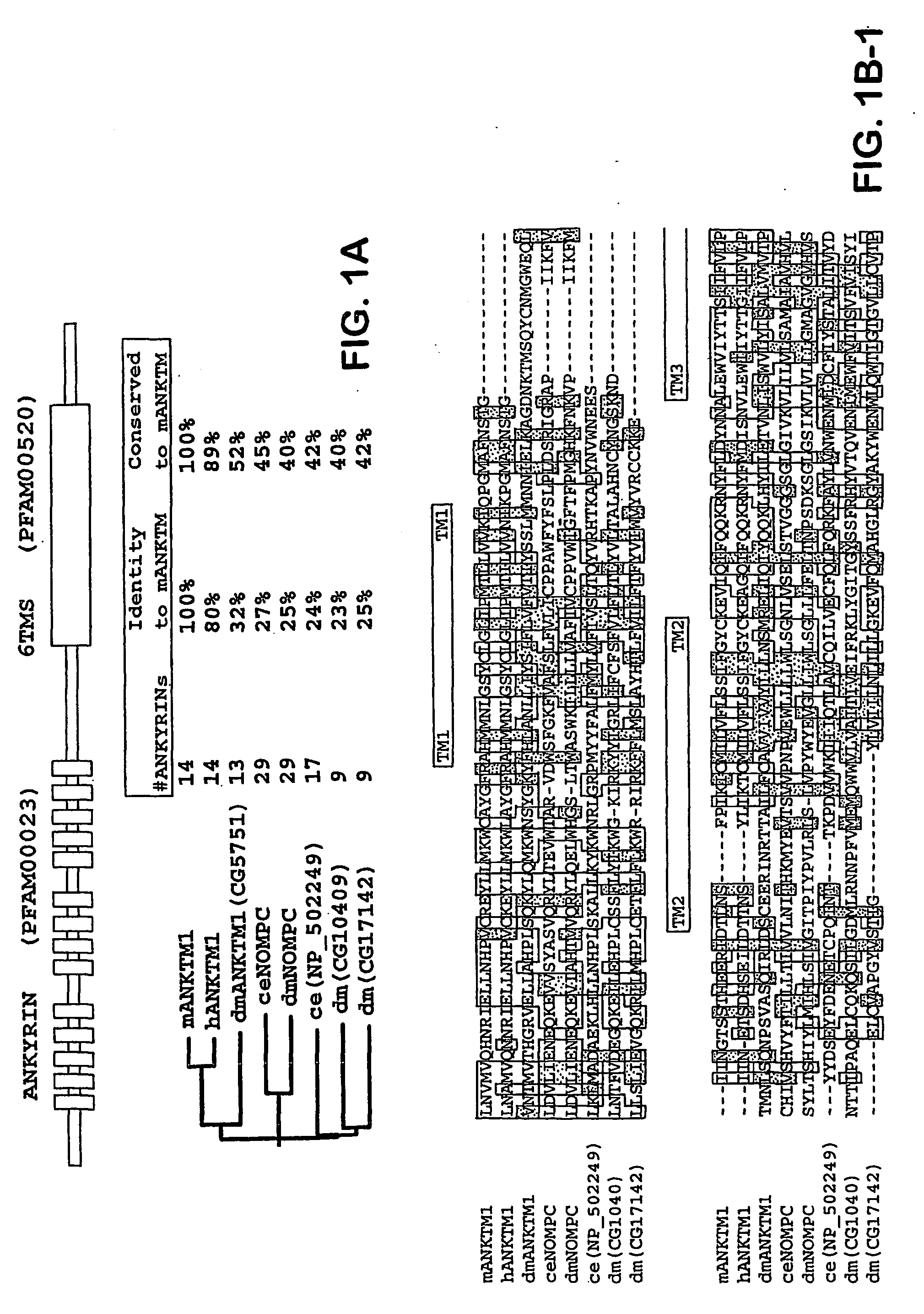
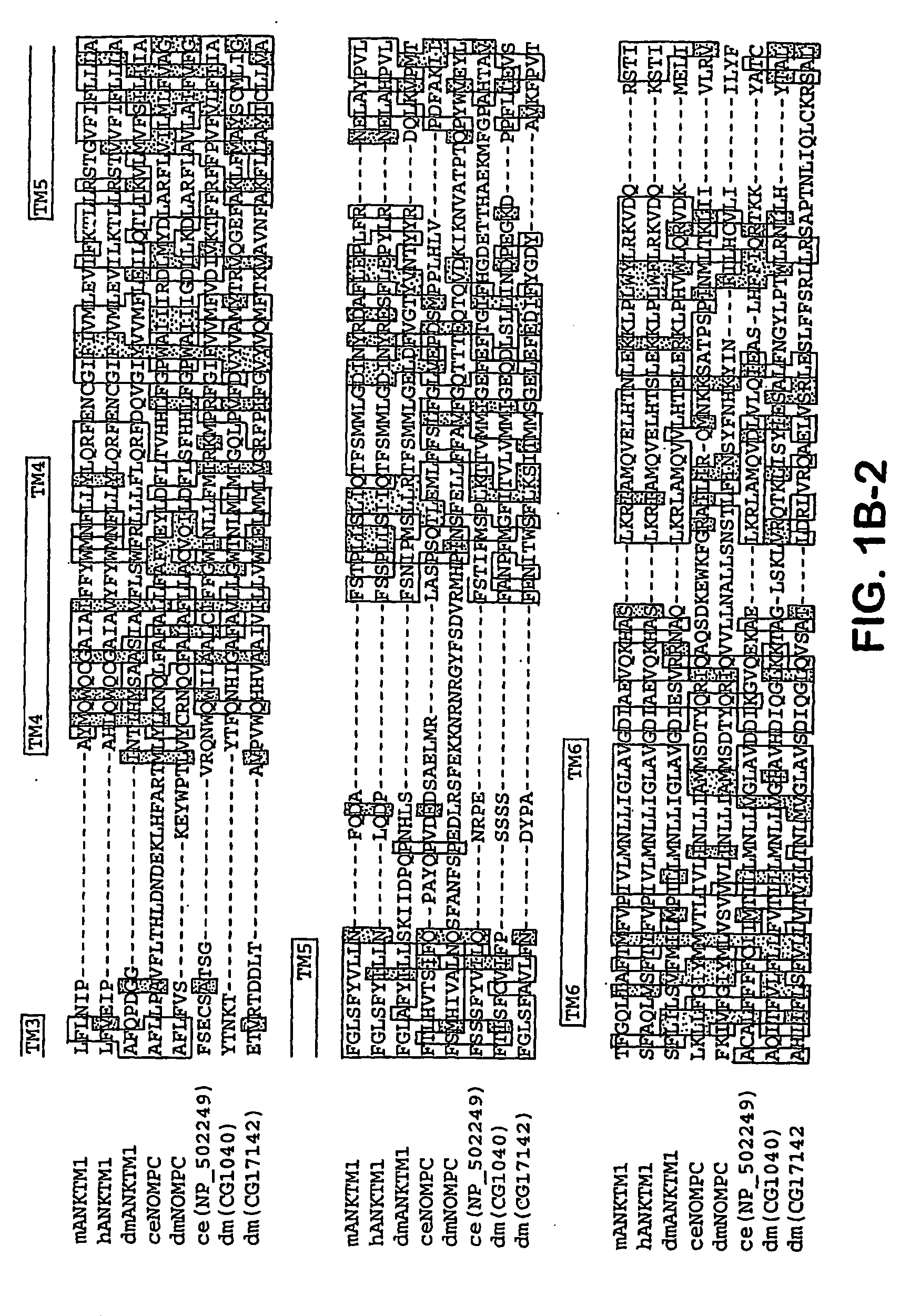
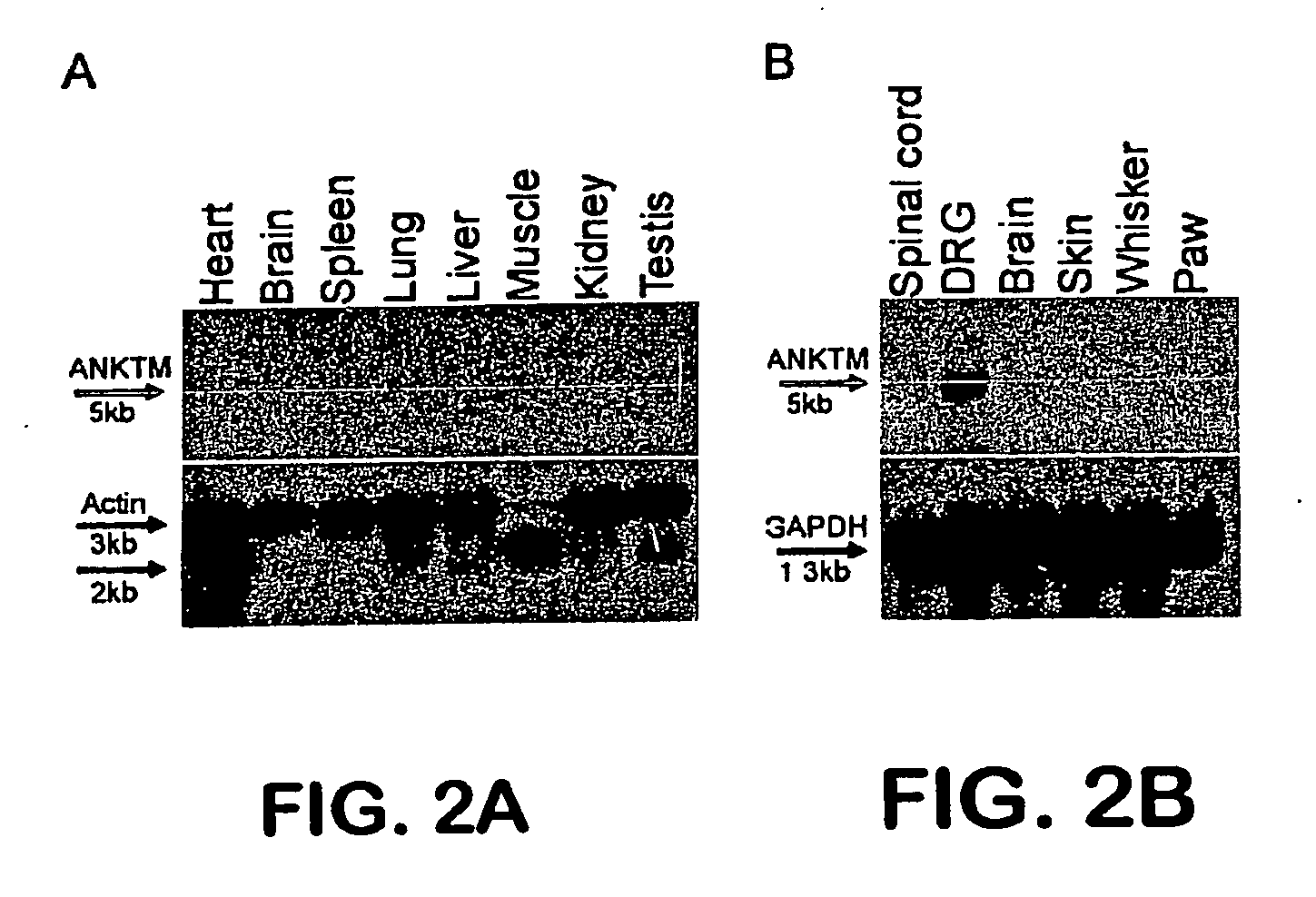
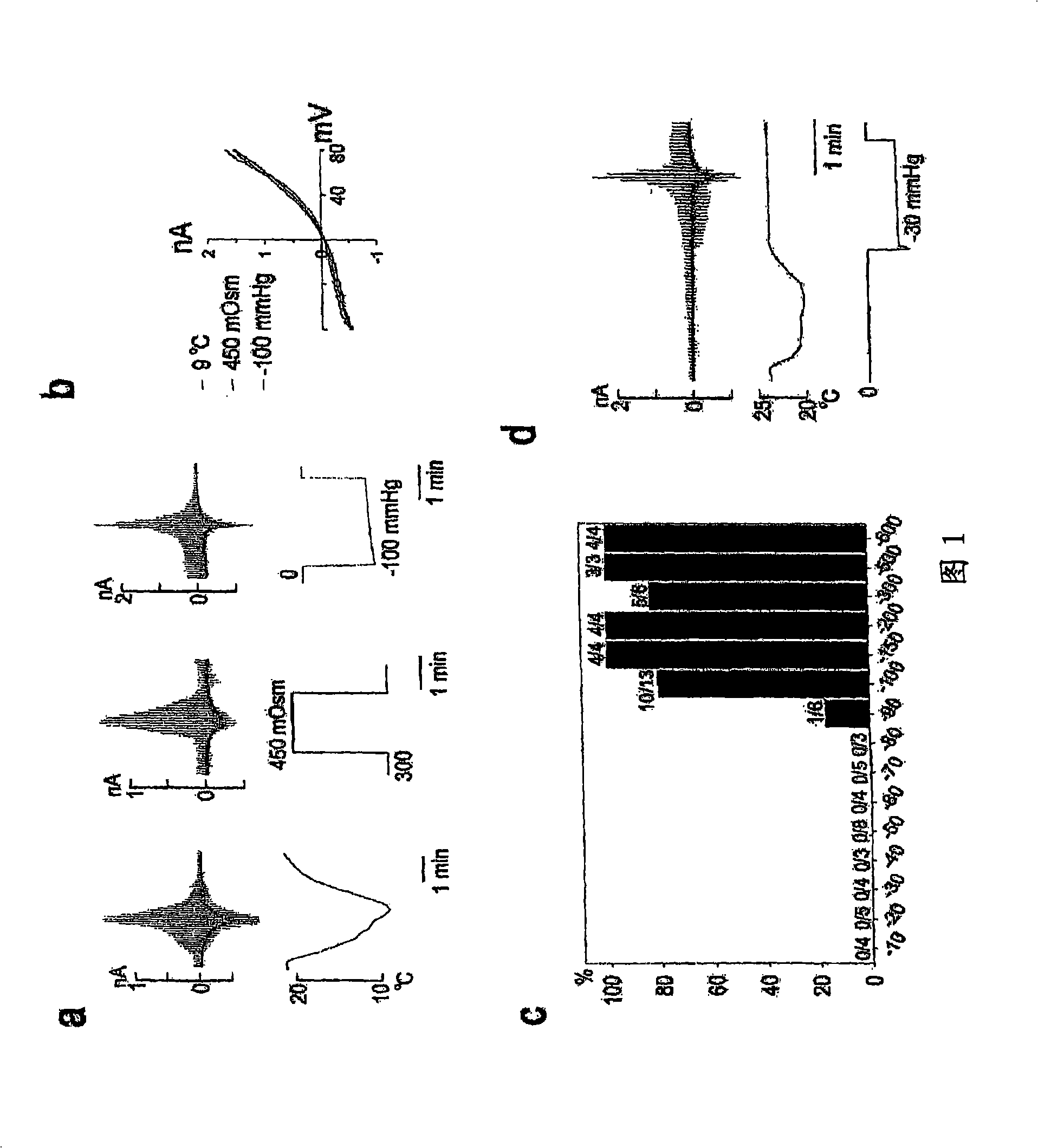
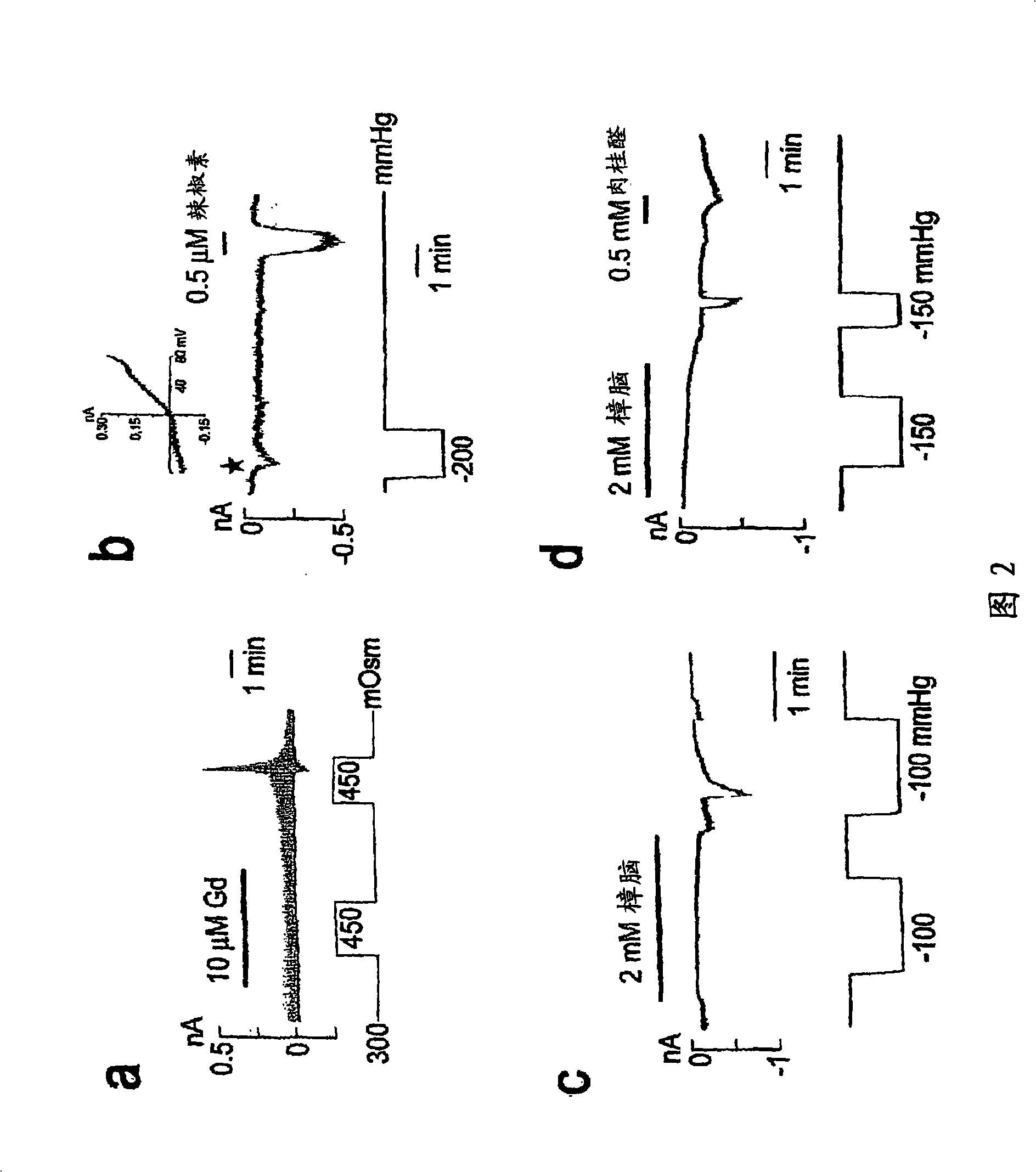
ANKTM1, a cold-activated TRP-like channel expressed in nociceptive neurons
The methods and compositions of the invention are based on a method for measuring nociceptive responses in vertebrates, including humans and other mammals utilizing a newly discovered thermoreceptor belonging to the Transient Receptor Potential (TRP) family of non-selective cation channels that participates in thermosensation and pain. This receptor, designated ANKTMI, is associated with nociceptive pain, such as hyperalgesia. Accordingly, the invention provides isolated polypeptides and polynucleotides associated with nociception as well as methods for identifying or screening agents that modulate nociception.
Owner:NOVARTIS AG +1
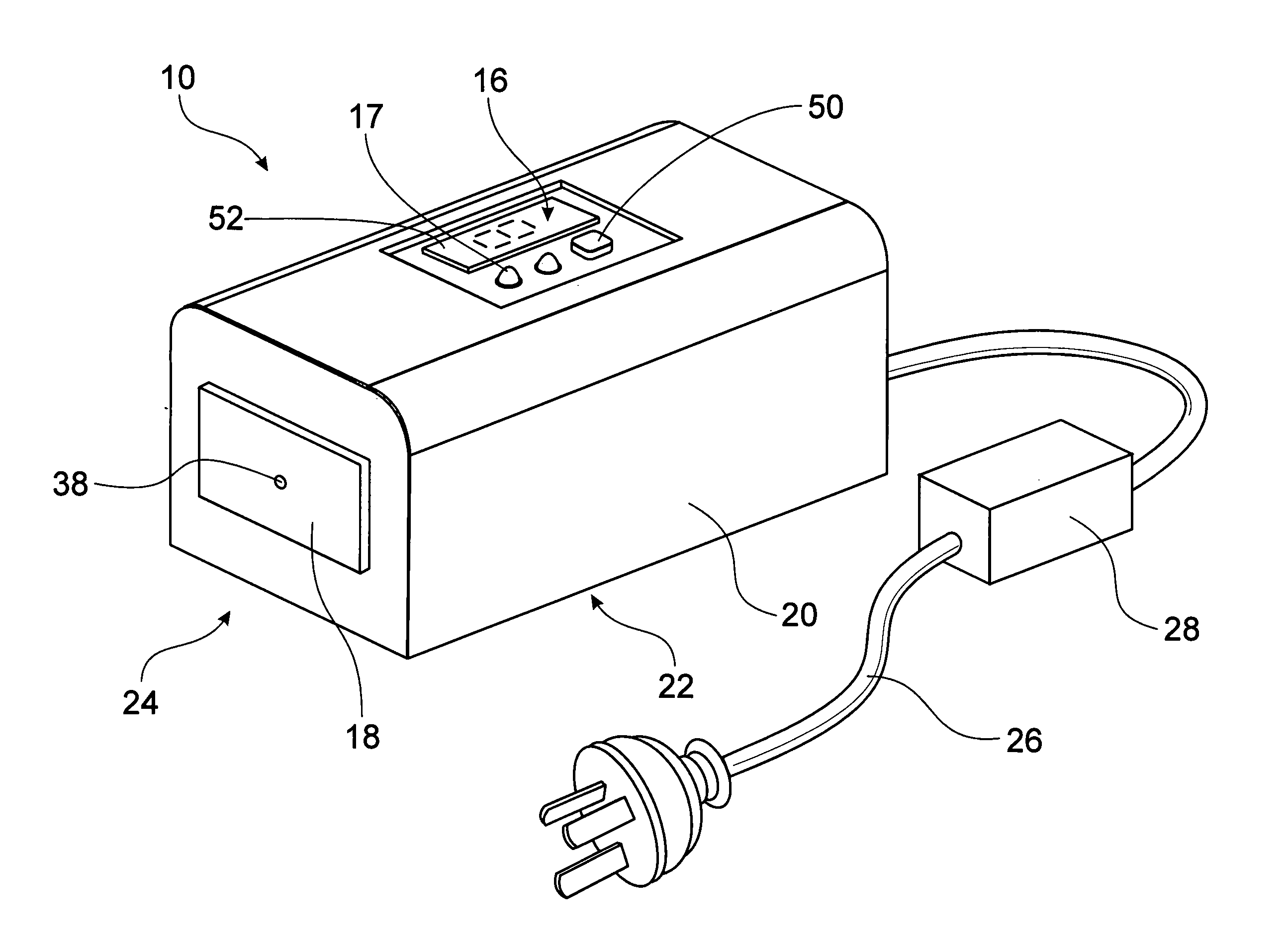
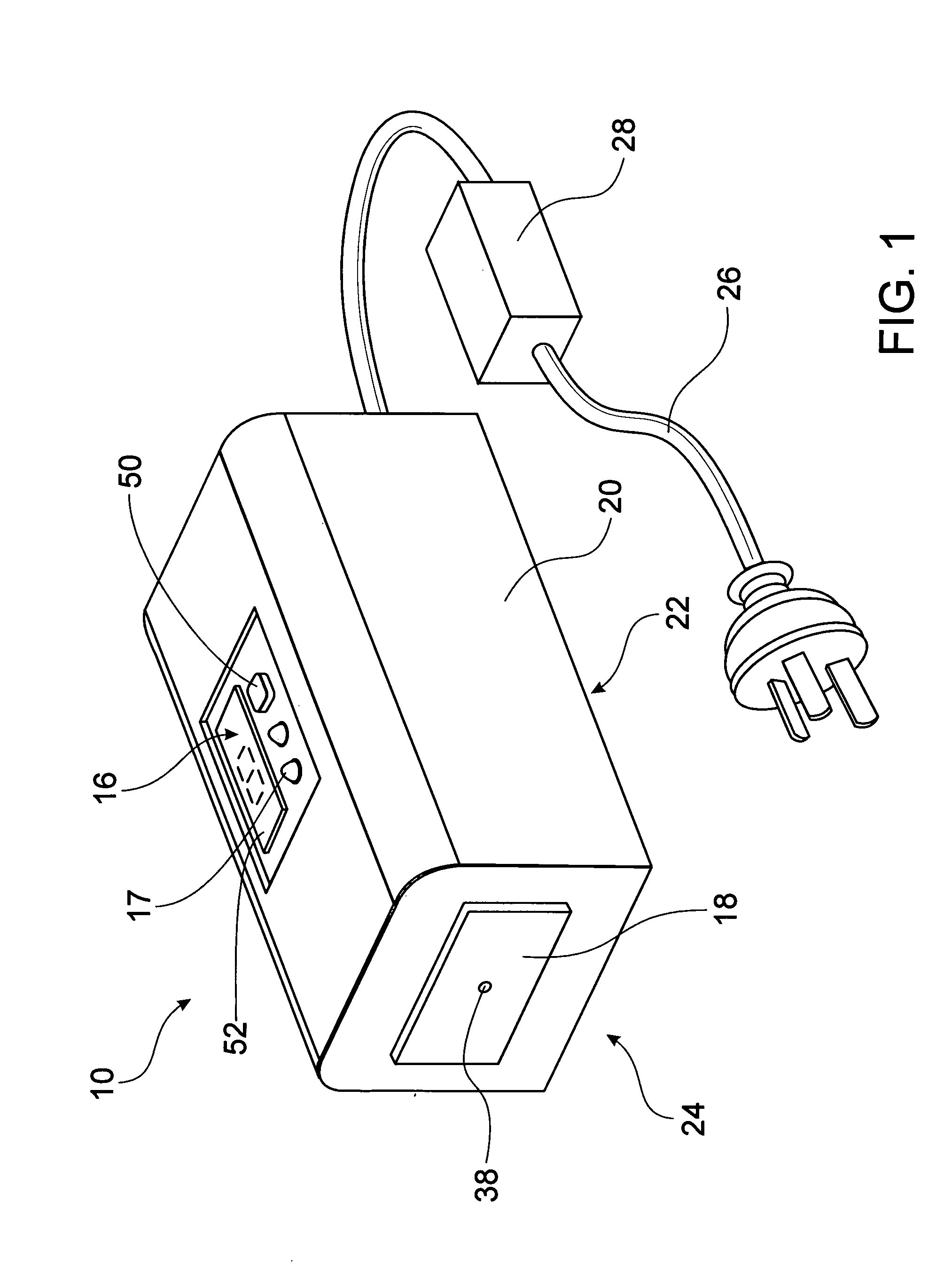
Thermo-electric device
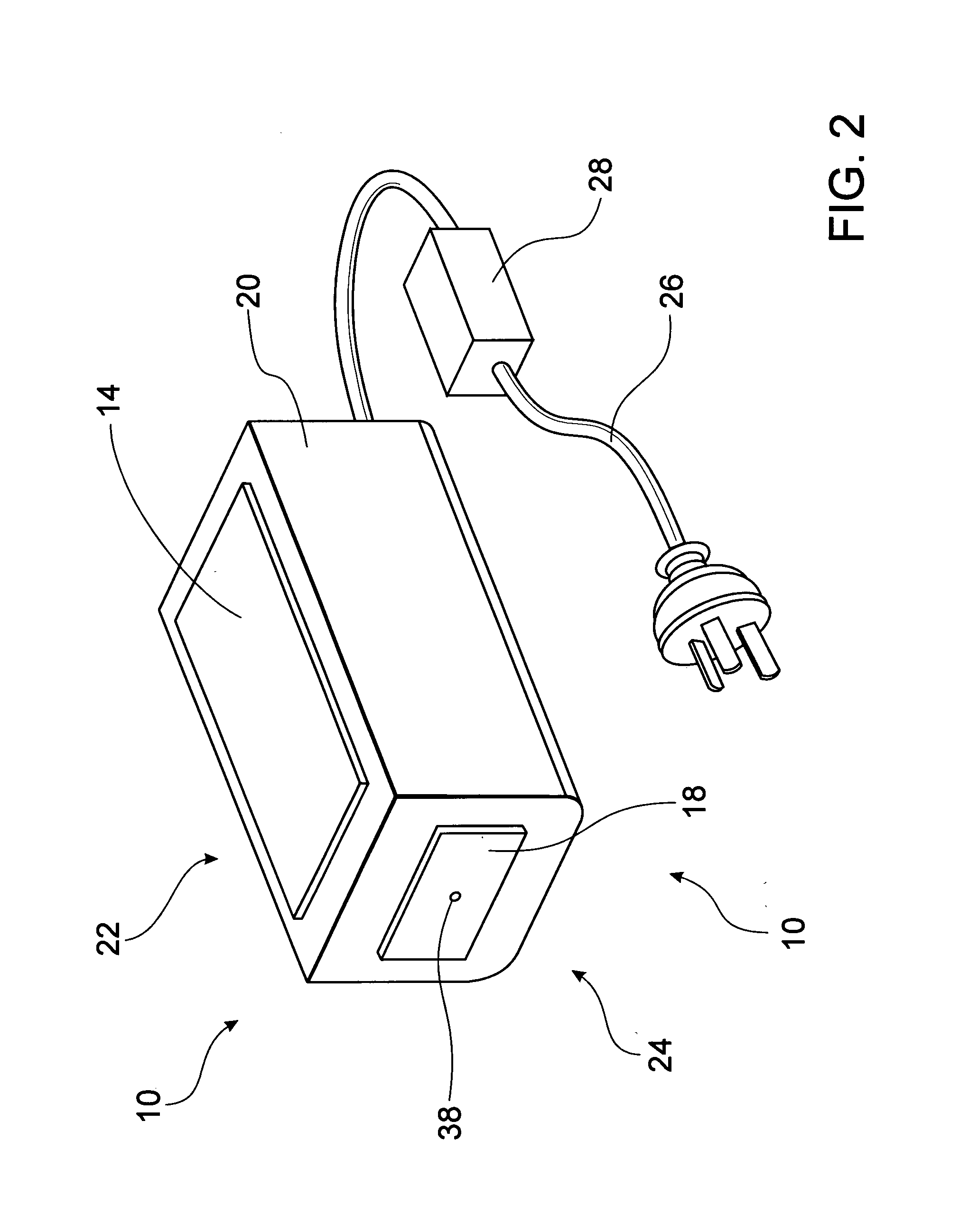
A portable handheld thermo-electric device (10) is used to test for decreased cold pain thresholds or increased sensitivity to cold stimulation (cold hyperalgesia) in a patient. The thermo-electric device (10) comprises a probe (18), a Peltier module (12) and a control unit (16) operable to energize the Peltier module (12) so that the temperature of the probe 18 is variable in a range between a predetermined upper temperature limit and a predetermined lower temperature limit during a test cycle of the thermo-electric device (10). A thermal mass (14) is adapted for thermal contact with an interface side (34) of the Peltier module (12). The Peltier module (12) has thermal properties which enables it to be cooled to a temperature below the predetermined upper temperature limit and thereafter maintain the interface side (34) of the Peltier module (12) at a temperature below the predetermined upper temperature limit for the duration of the test cycle.
Owner:THE UNIV OF QUEENSLAND
Trp inhibitors and uses thereof
The present invention, relates to methods including compounds, derivatives, antibodies, interfering RNA, biologies, polypeptides, dominant negative effectors, and their use in the treatment of neuropathic pain by inhibition of transient receptor potential (TRP) channels. In another embodiment, this invention relates to inhibitors, antagonists, and agonists of TRPC4. TRPC4 therapeutic agents and modulators include but are not limited to small molecule inhibitors, compounds, amino acid derivatives, polypeptides, RNA interference agents, natural chemicals, ligand derivatives, and ions. TRPC4 therapeutic agents and modulators are developed for the treatment of neuropathic pain, including but not limited to pain sensations such as nociception, hyperalgesia, allodynia, and loss of sensory function.
Owner:POSEIDA THERAPEUTICS INC
Anktm1, a cold-activated trp-like channel expressed in nociceptive neurons
InactiveUS20060142547A1Reducing nociceptive painReduce painNervous disorderBacteriaThermoreceptorMammal
The methods and compositions of the invention are based on a method for measuring nociceptive responses in vertebrates, including humans and other mammals utilizing a newly discovered thermoreceptor belonging to the Transient Receptor Potential (TRP) family of non-selective cation channels that participates in thermosensation and pain. This receptor, designated ANKTMI, is associated with nociceptive pain, such as hyperalgesia. Accordingly, the invention provides isolated polypeptides and polynucleotides associated with nociception as well as methods for identifying or screening agents that modulate nociception.
Owner:NOVARTIS AG +1
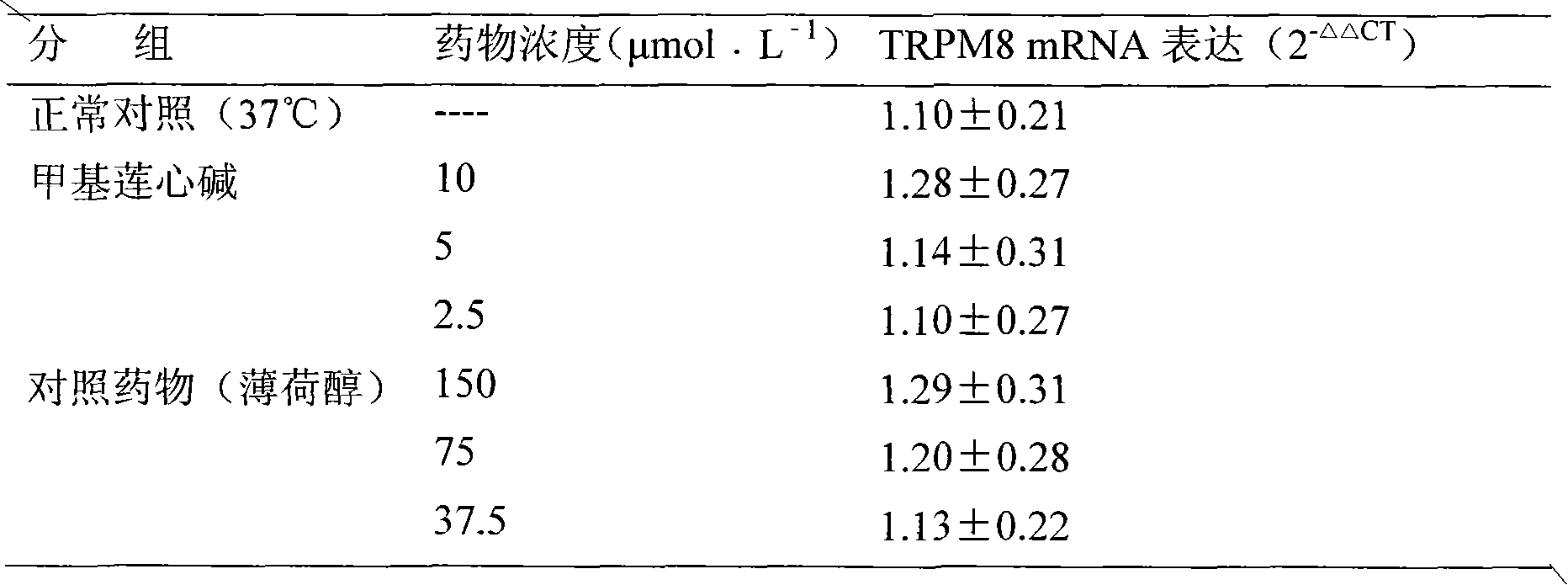
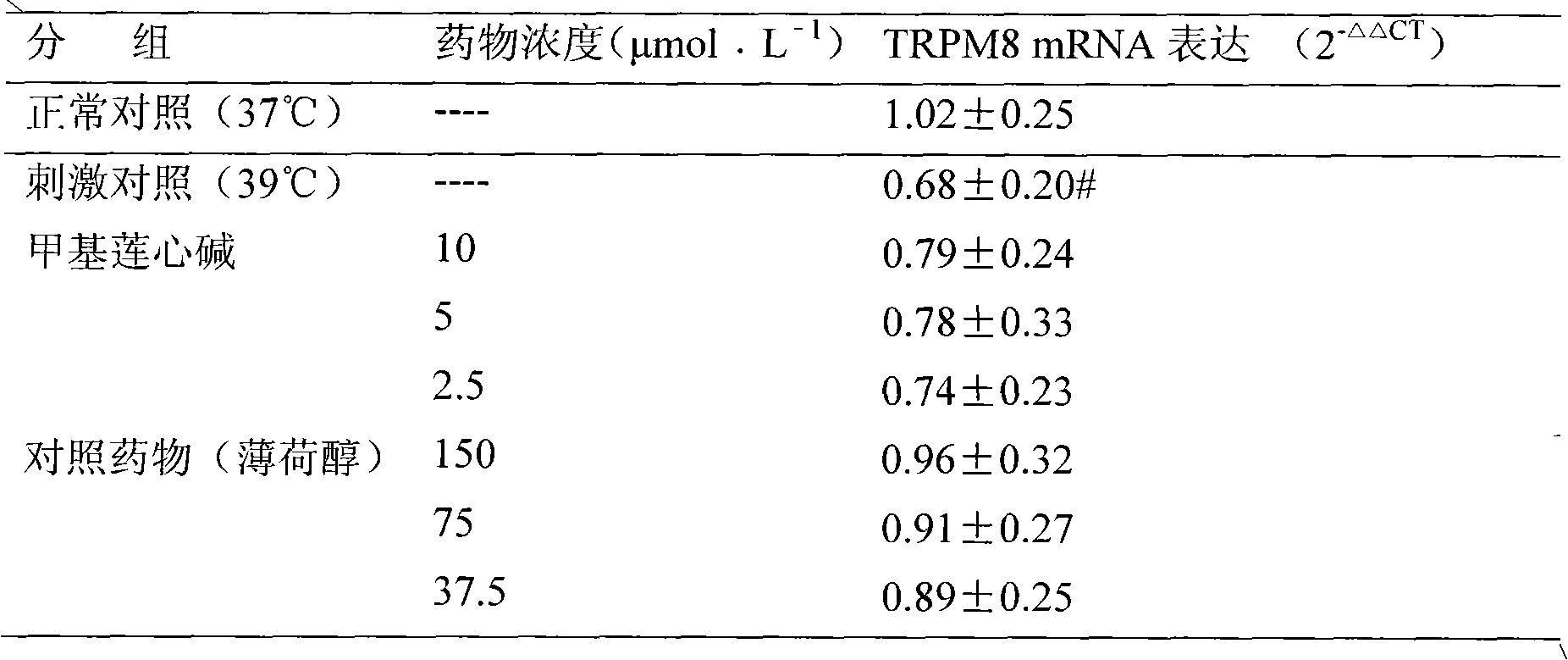
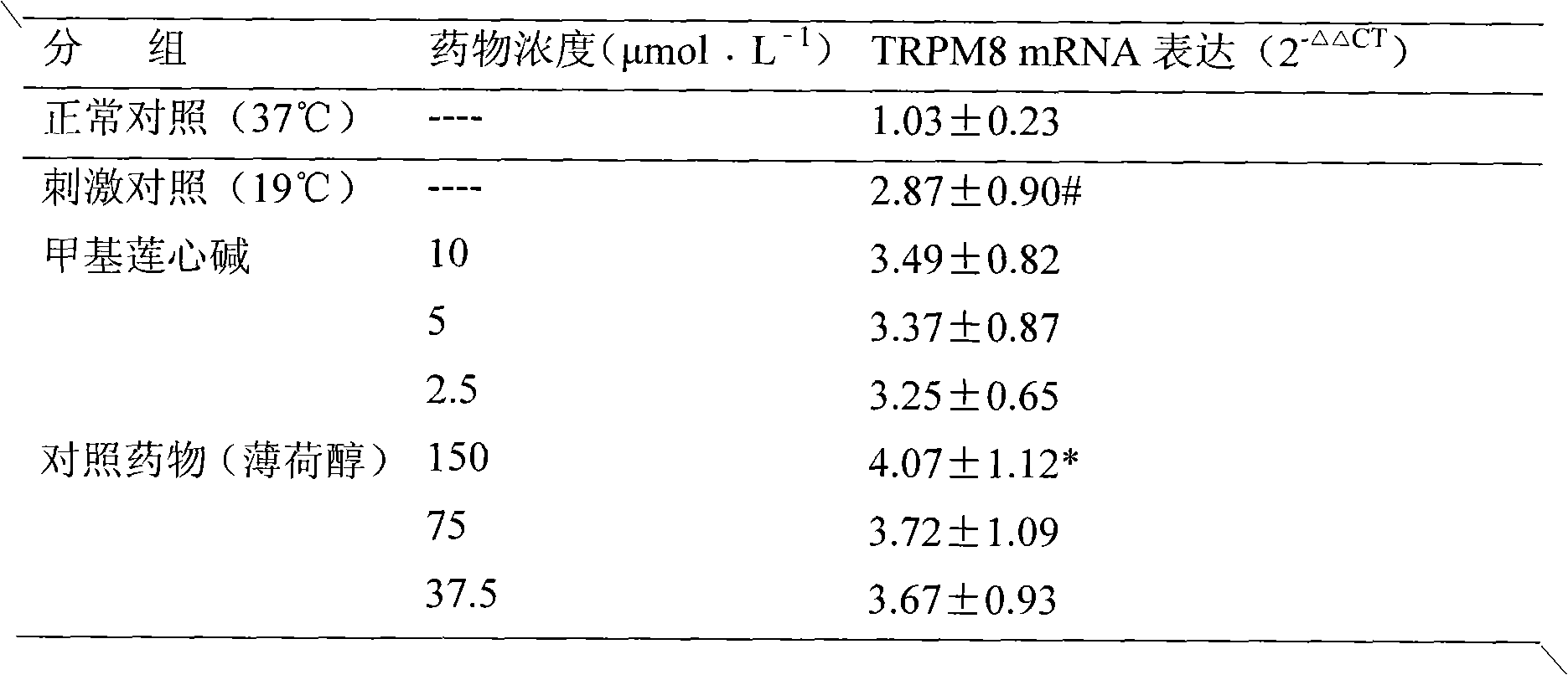
New use of methyl liensinine
ActiveCN101862331AIncrease the strength of actionStrong creativityOrganic active ingredientsNervous disorderWilms' tumorHyperalgesia
The invention discloses a new use of methyl liensinine. Methyl liensinine respectively regulates M8 and V1 subtype transient receptor potential ion channels (TRPM8 and TRPV1 for short) of mammal including human, so as to prepare medicine for treating related diseases joined by the ion channels (such as cold hyperalgesia, parkinsonism, nociceptive bladder syndrome, chronic obstructive lung diseaseand tumours of skin, prostate, breast, lung and colon). The action strength of methyl liensinine of the invention is higher than that of menthol.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
Compositions and methods for inhibition of phospholipase a2 mediated inflammation
Specific, highly potent 2-oxo-amide based inhibitors of phospholipase A2 (PLA2) activity are provided. A role for PLA2 activity in spinally mediated inflammatory processes is established, and a method for treating hyperalgesia and other inflammatory conditions associated with PLA2 activity is provided.
Owner:RGT UNIV OF CALIFORNIA
Apparatus and method for human algometry
An apparatus and method for performing human algometry are disclosed. They include a stimulator configured to apply electrical stimulation of variable intensity to an area of a patient's body, a monitoring device configured to measure a level of cortical activity in one or more regions of the patient's brain, and a microprocessor connected to the stimulator and the monitoring device that is configured to correlate the intensity of the electrical stimulation with the level of activity in the one or more regions of the patient's brain and to determine at least one of a measurement of pain intensity, a measurement of a sensory detection threshold (SDT), a measurement of a drug's analgesic impact, an indication of an onset of tolerance to a drug, an indication of an onset of analgesic-induced hyperalgesia, an indication of conditions of allodynia, a measurement of dose-response characteristics of pain management drugs, and a characterization of a pain condition.
Owner:CHILDRENS NAT MEDICAL CENT
Device and method for assessing operant facial pain
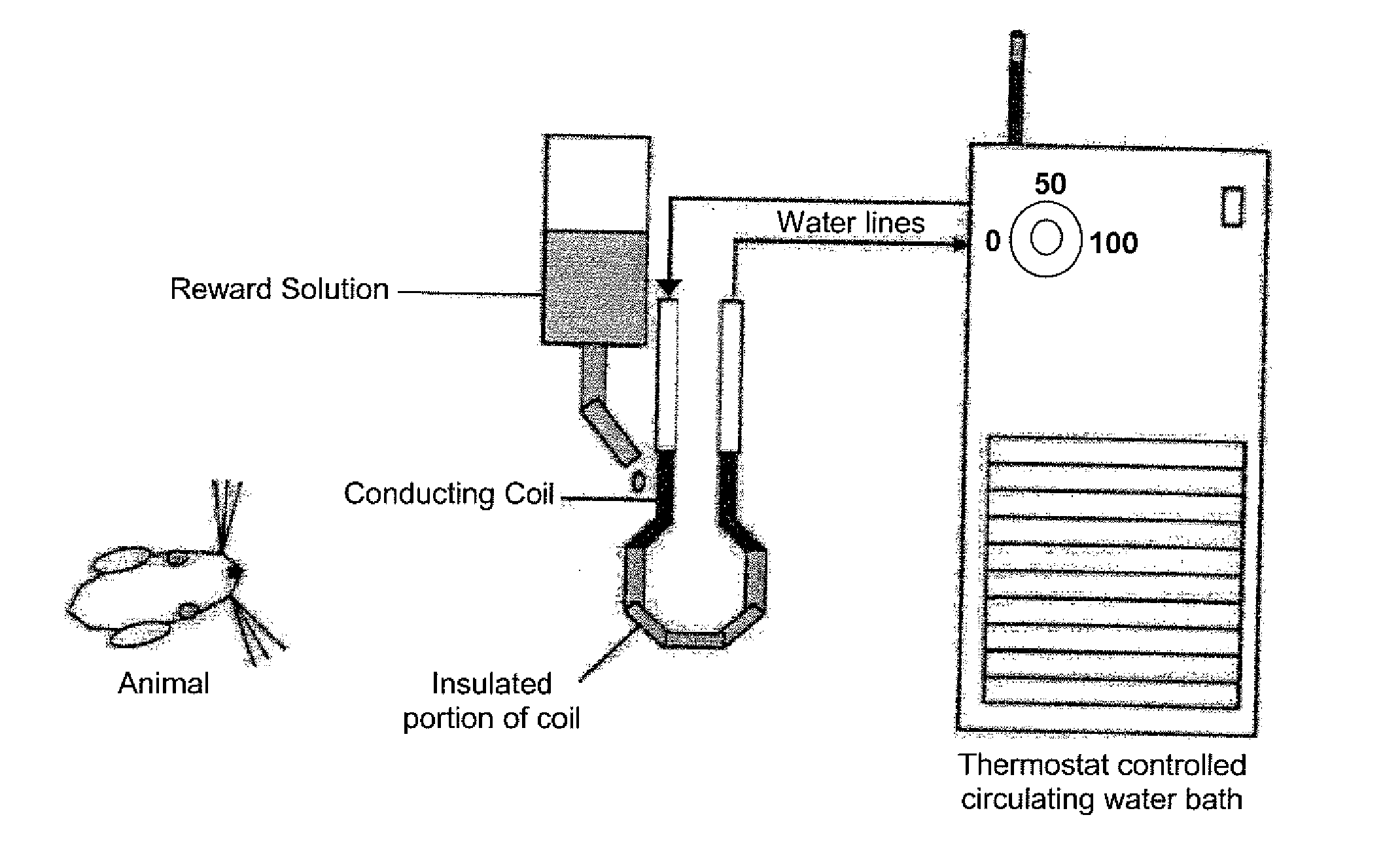
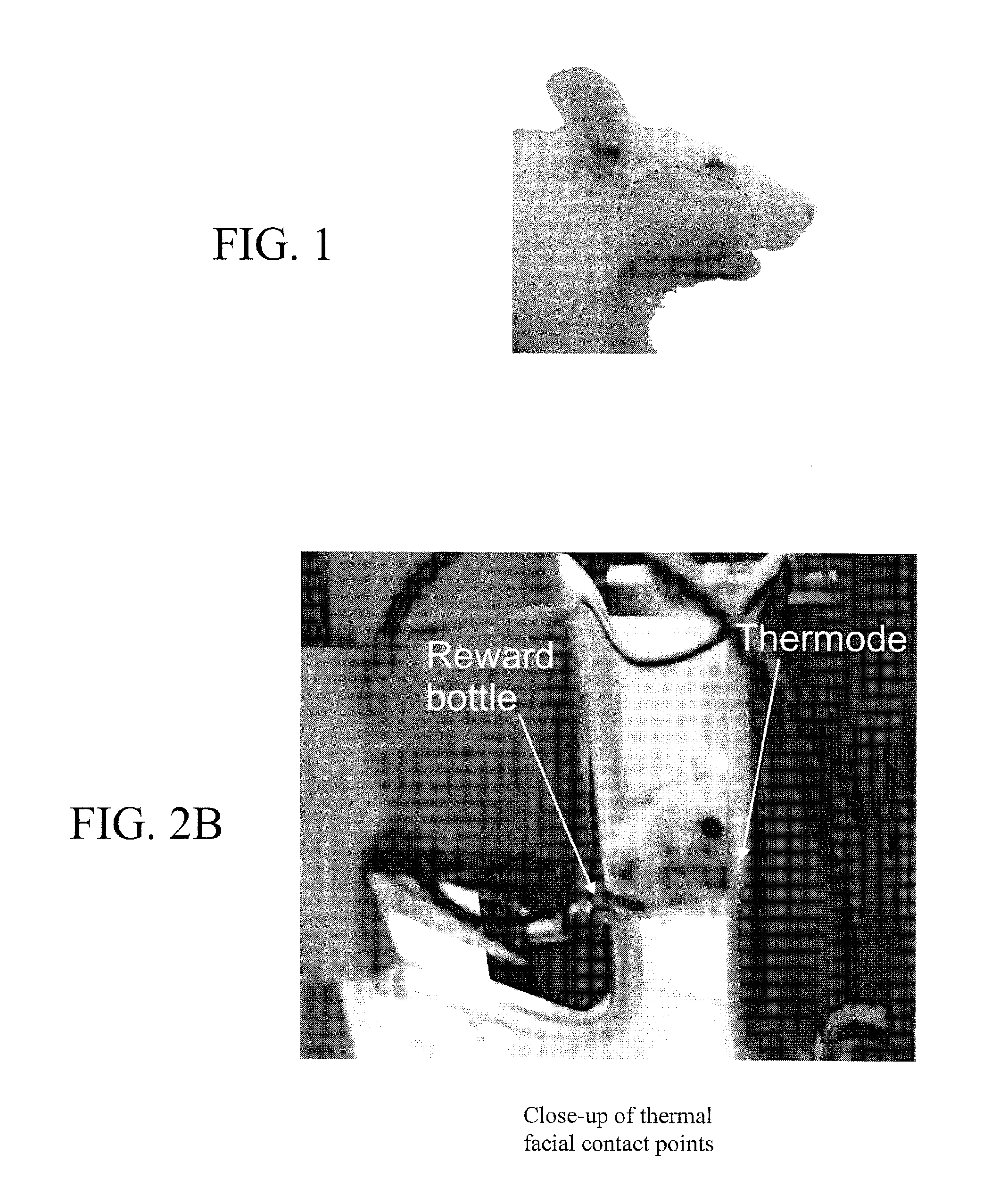
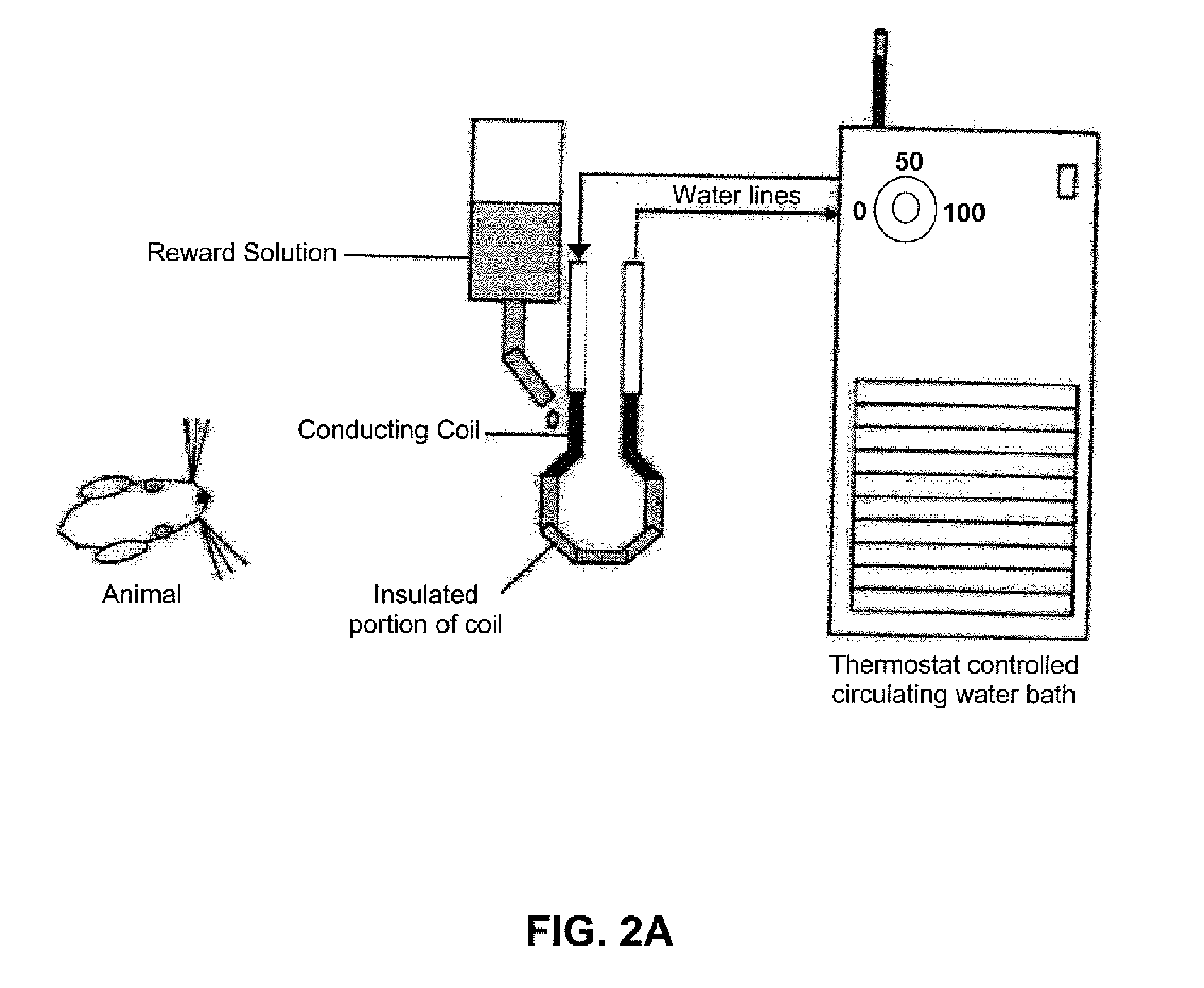
InactiveUS20100331722A1Increase experienceImprove processingSurgeryAnimal housingOrofacial painFacial region
The subject invention concerns a method and device for assessing facial pain sensitivity exhibited by an animal. The device and method can be used, for example, to evaluate the effect of a disease state, drug, or other intervention, on facial pain sensitivity, such as orofacial pain sensitivity. In one embodiment, the device and method provide a way of assessing both heat and cold sensitivity (hyperalgesia and allodynia) in the facial region in a non-invasive manner. Additionally, since the animals can be kept unrestrained, there are less confounding factors such as stress, which are inherent to other facial pain testing techniques.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Antagonists of the Vanilloid Receptor Subtype 1 (VR1) and Uses Thereof
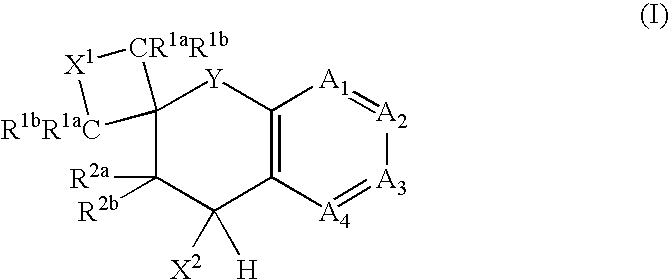
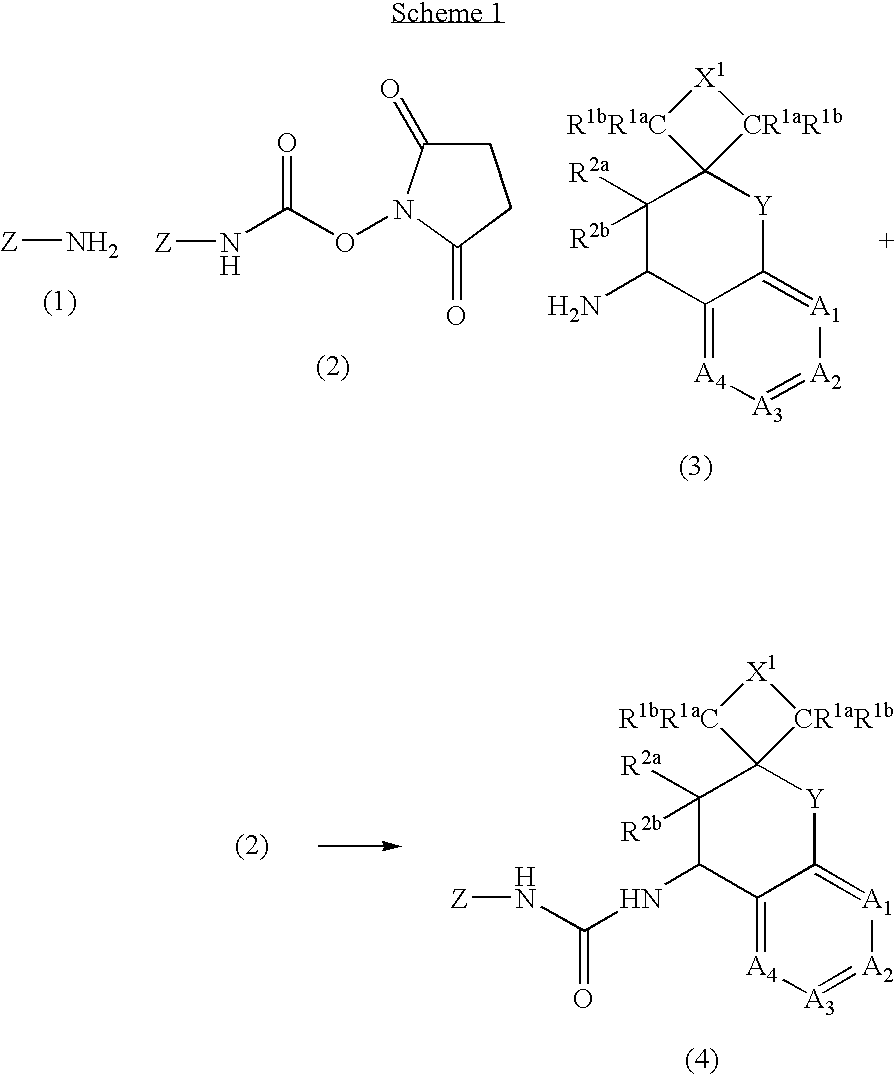
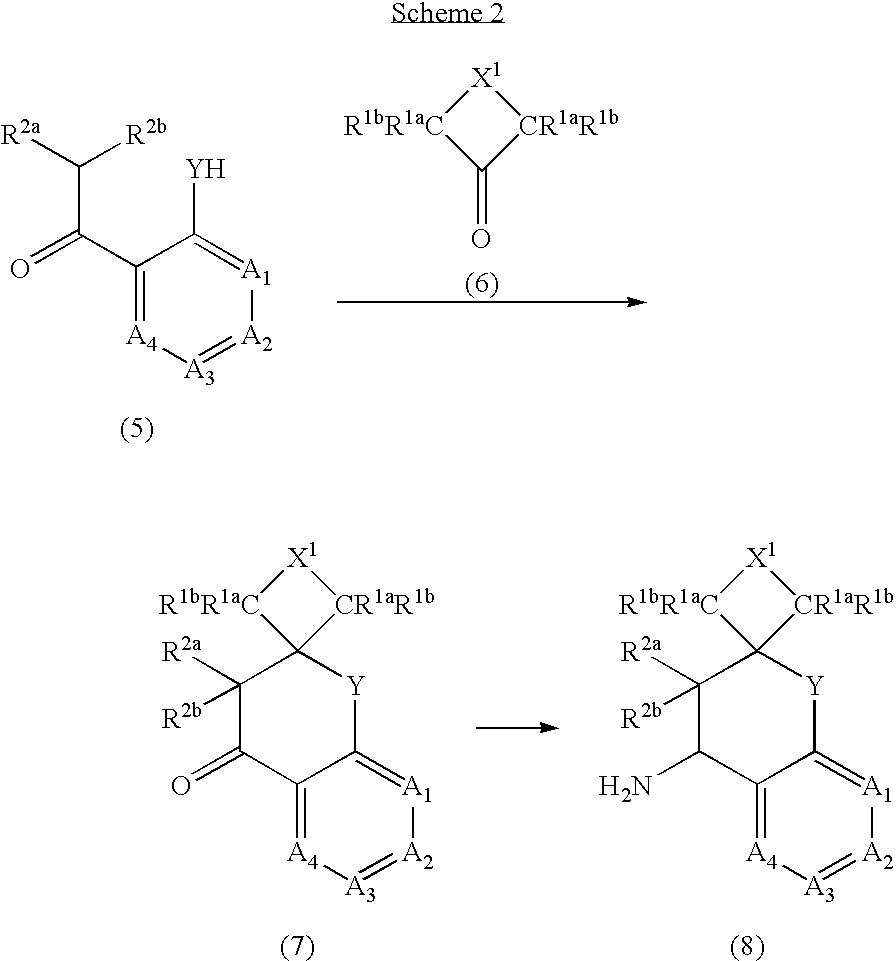
The present invention is directed to compounds of formula (I) wherein variables X1, X2, Y, R1a, R1b, R2a, R2b, A1, A2, A3, and A4 are as defined in the description, and methods of use to treat pain, neuropathic pain, allodynia, pain associated with inflammation or an inflammatory disease, inflammatory hyperalgesia, bladder overactivity, and urinary incontinence.
Owner:ABBVIE INC
Treatment of allodynia, hyperalgesia, spontaneous pain and phantom pain
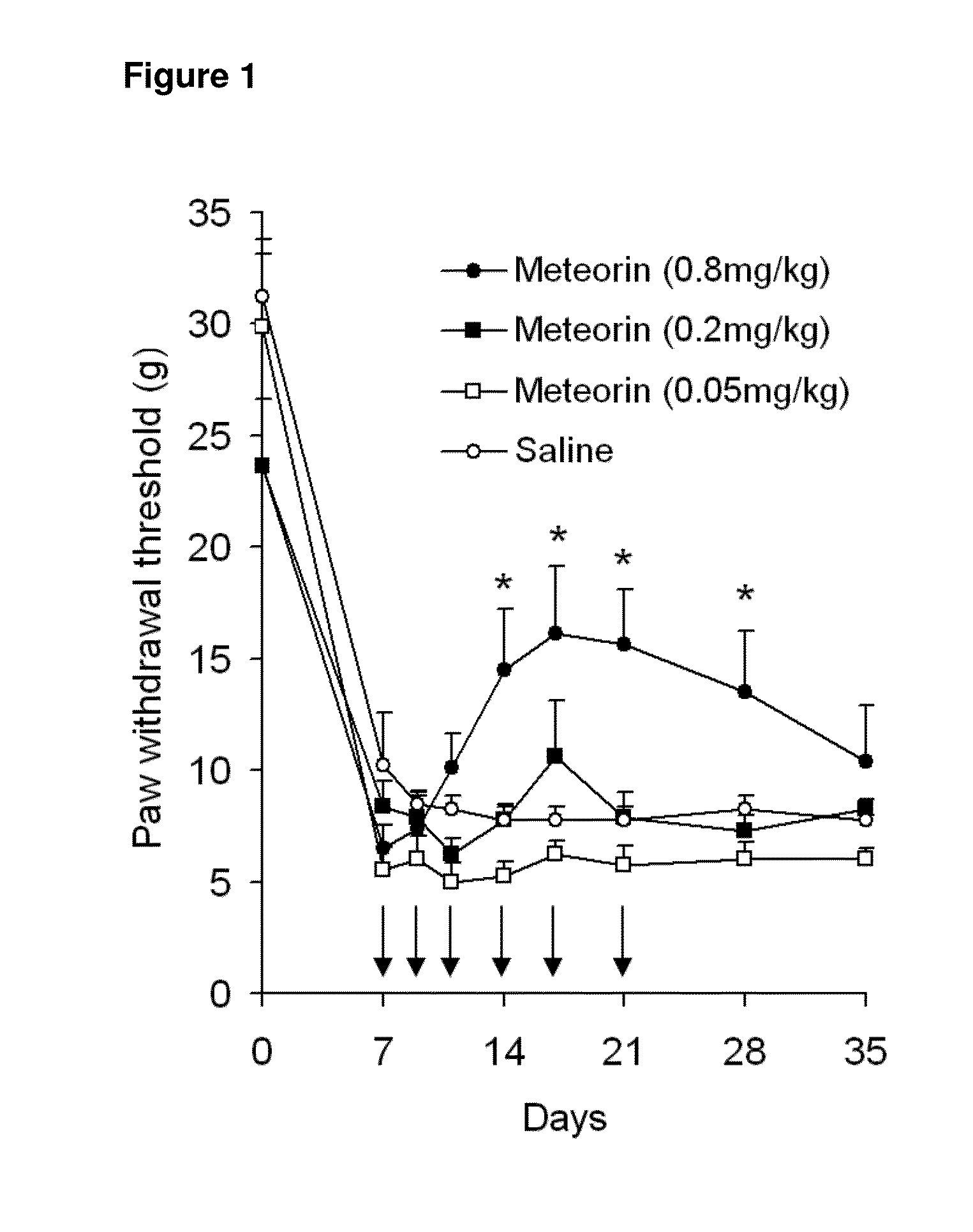
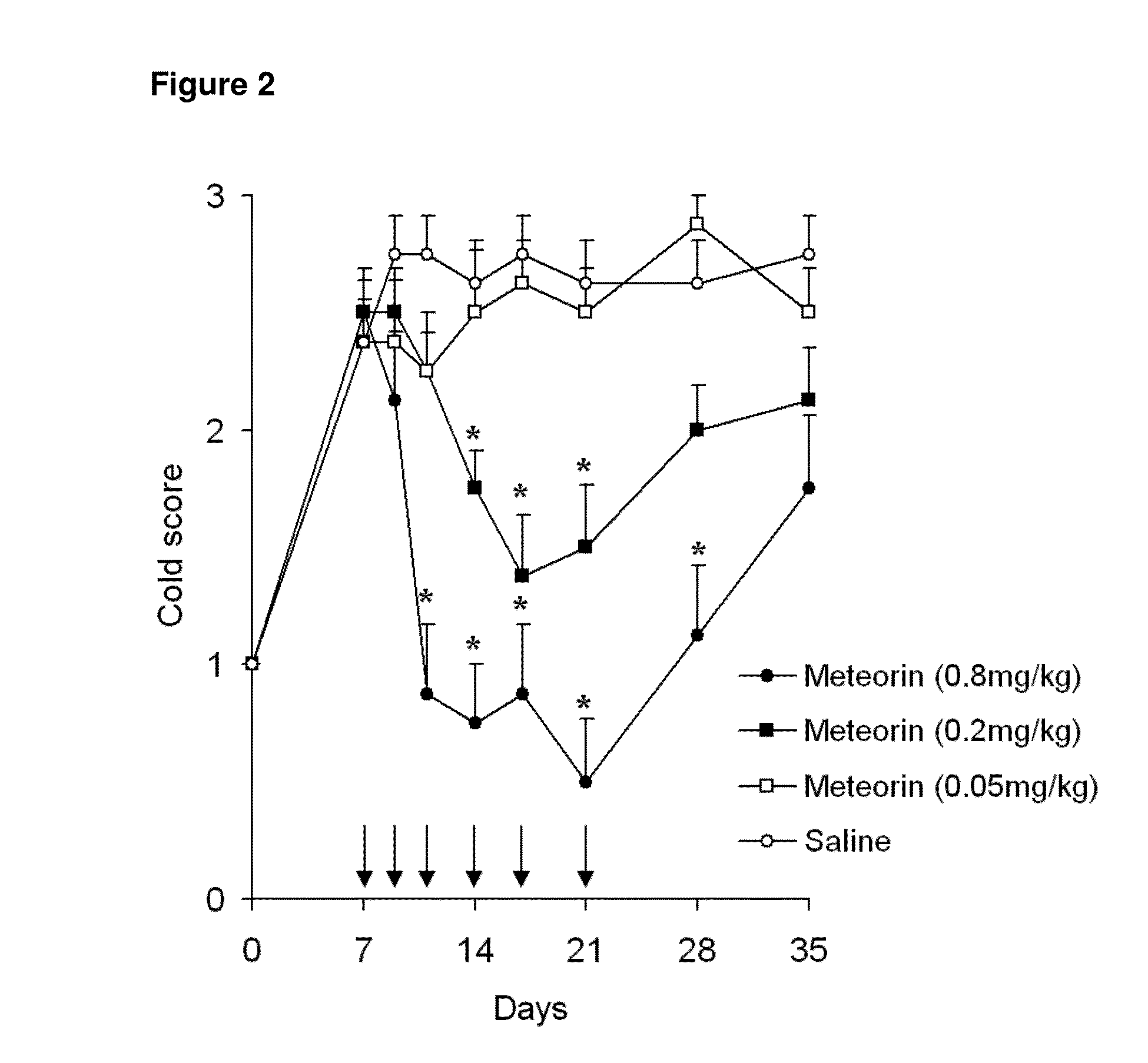
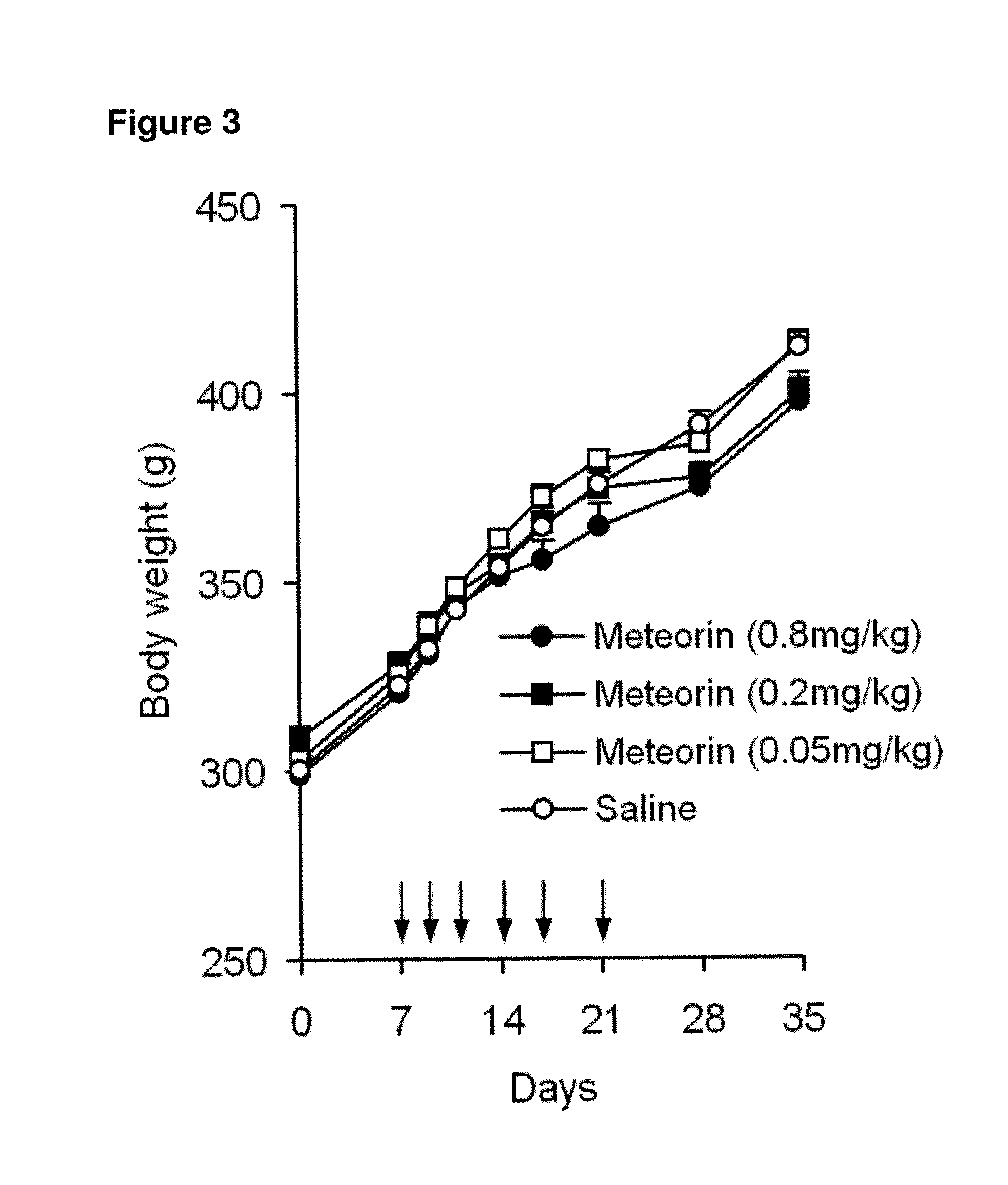
The present invention relates to the use of Meteorin for the treatment of allodynia, hyperalgesia, spontaneous pain and phantom pain. In a preferred embodiment the disorder to be treated is allodynia, and hyperalgesia, more preferably allodynia including thermal and tactile allodynia.
Owner:HOBA THERAPEUTICS APS
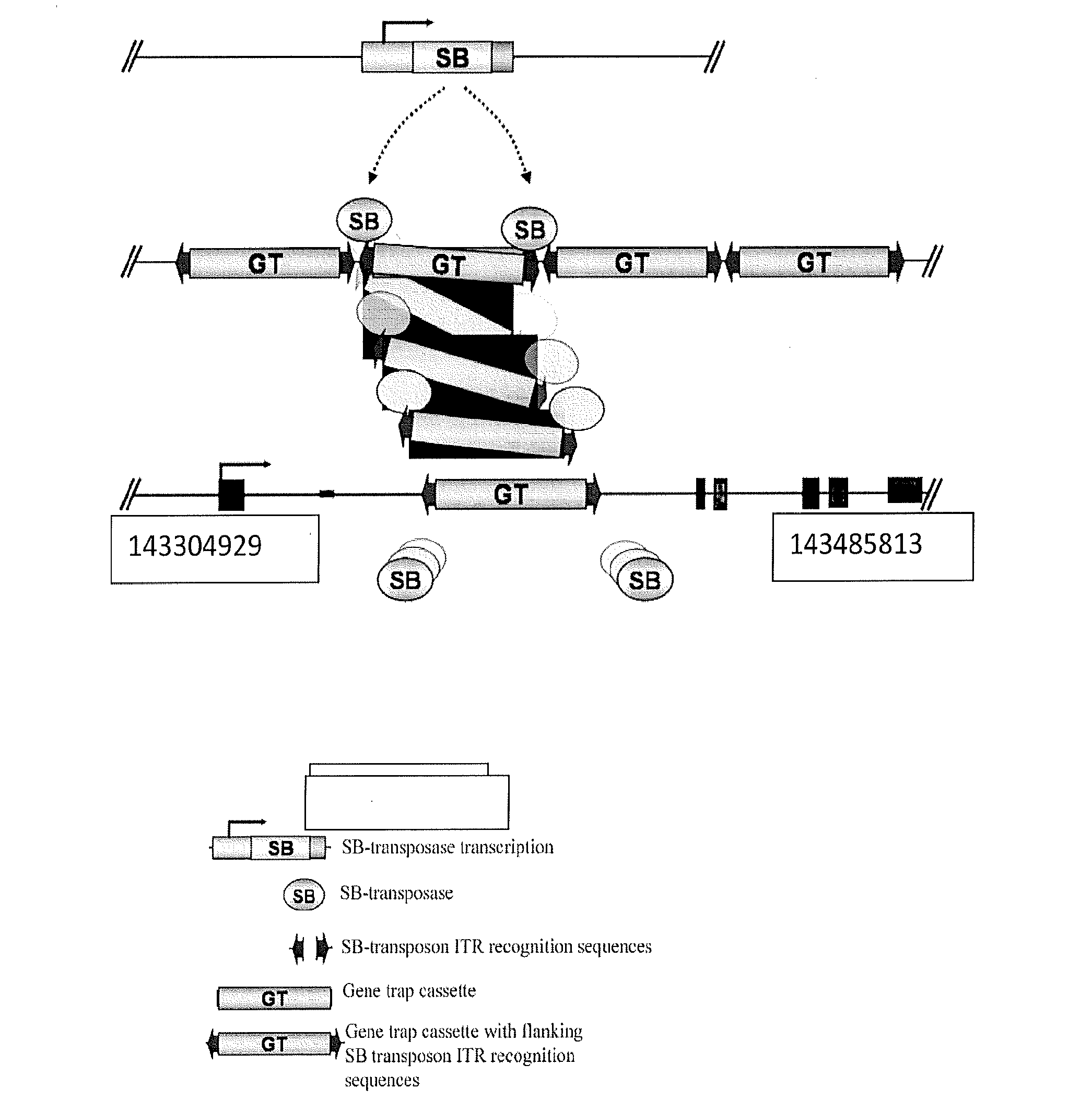
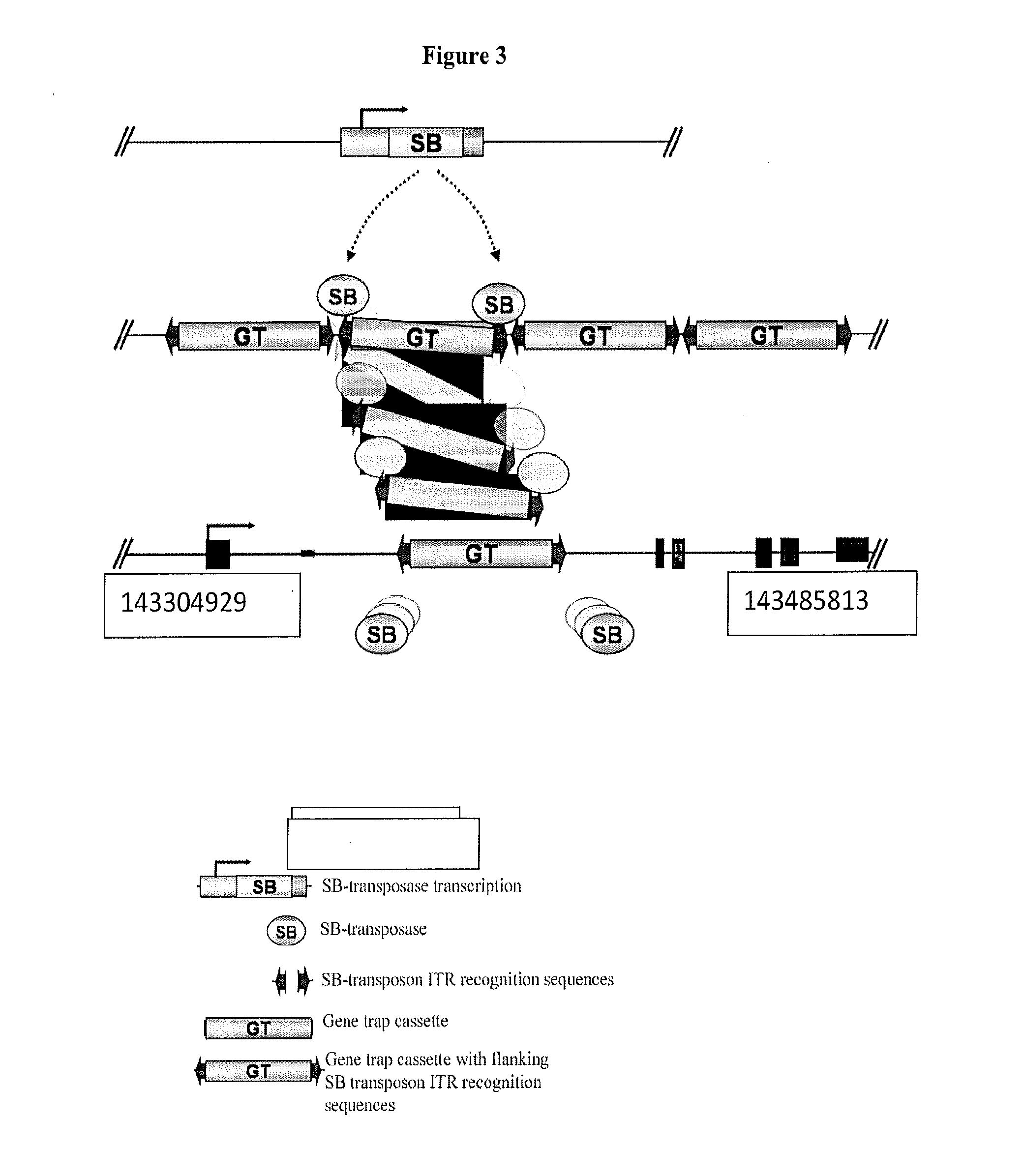
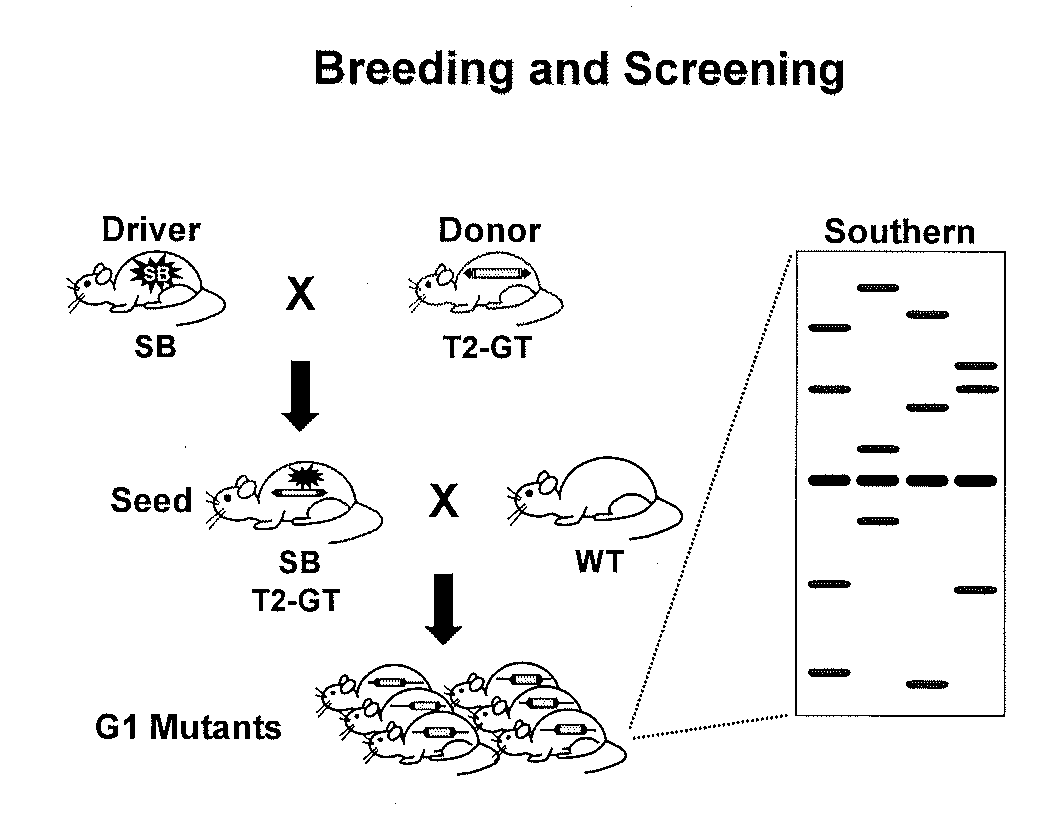
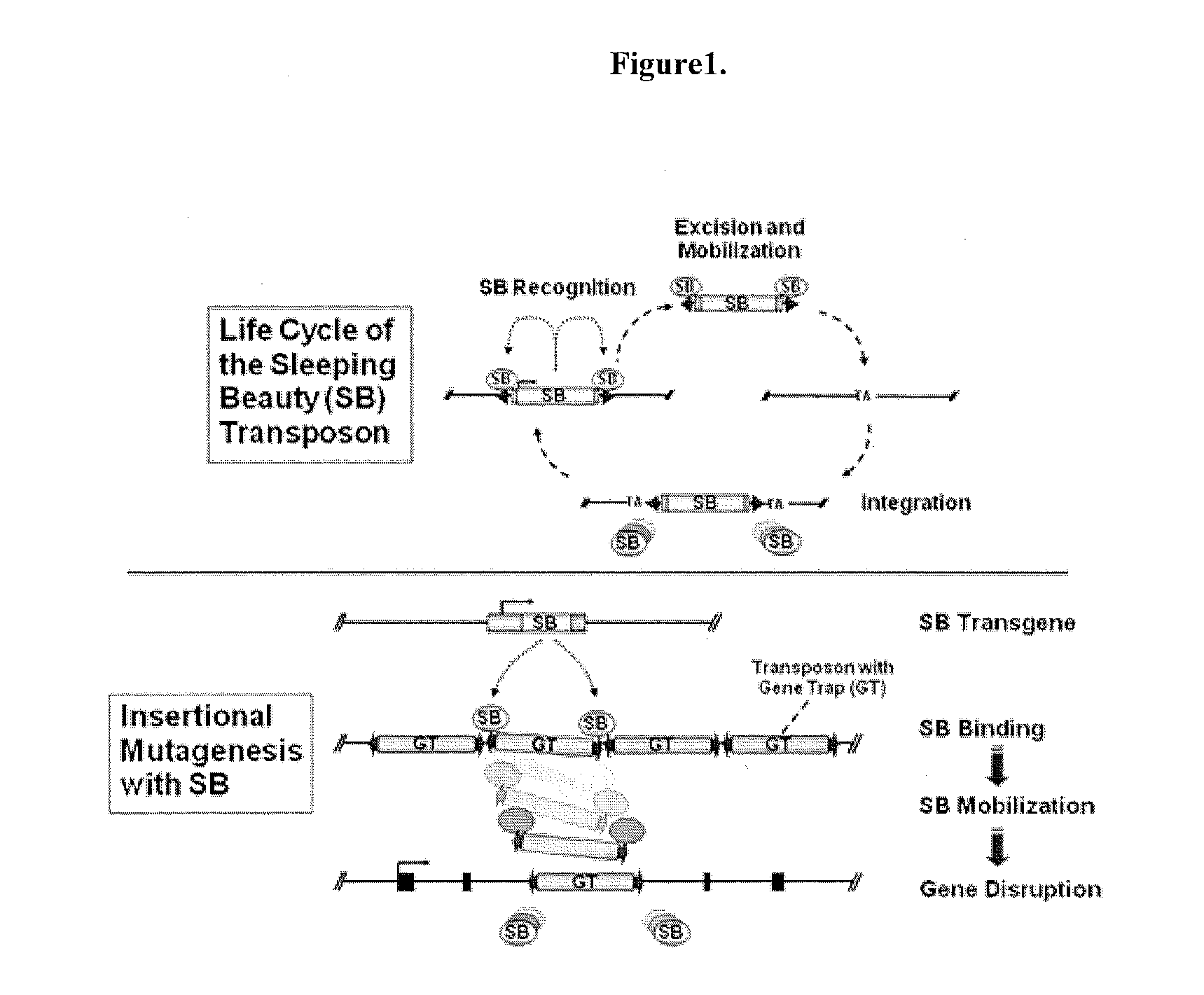
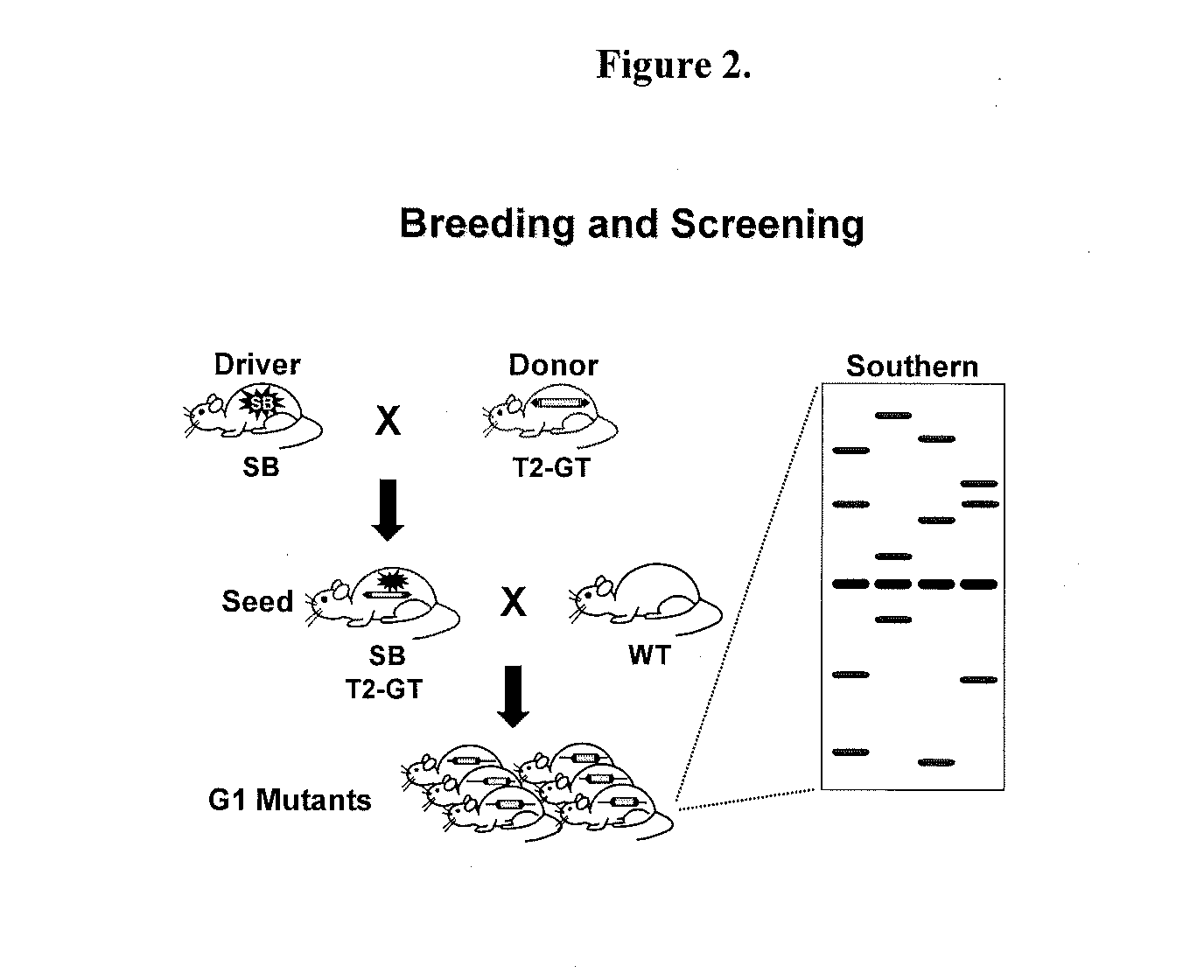
Genetically Modified Rat Models for Pain
This invention relates to the engineering of animal cells, preferably mammalian, more preferably rat, that are deficient due to the disruption of gene(s) or gene product(s) resulting in altered nervous system function. In one aspect, the altered function results in pain in the mammal. In another aspect, the nervous system dysfunction results in prolonged hyperalgesia, allo dynia, and loss of sensory function. In another aspect, the invention relates to genetically modified rats, as well as the descendants and ancestors of such animals, which are animal models of altered nervous system function mediated pain and methods of their use. In another aspect, the genetically modified rats, as well as the descendants and ancestors of such animals, are animal models of nervous system dysfunction resulting in prolonged hyperalgesia, allodynia, and loss of sensory function and methods of their use. In another aspect, the present invention provides a method of identifying a compound useful for the treatment or prevention of pain.
Owner:TRANSPOSAGEN BIOPHARM
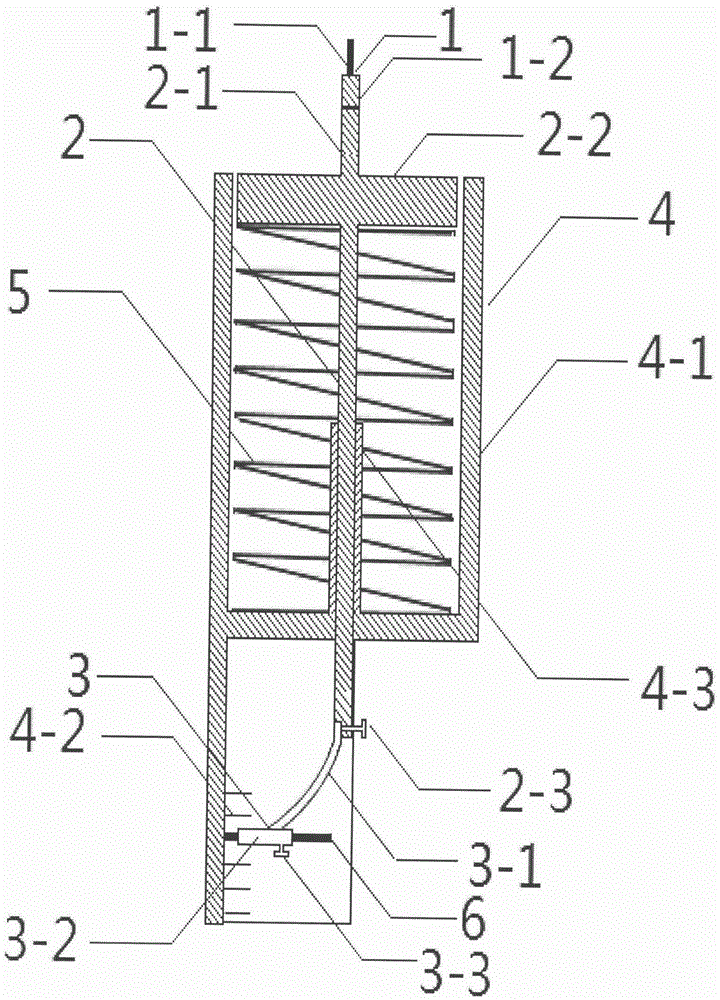
Portable mechanical hyperalgesia measuring apparatus
InactiveCN105476601AImprove availabilitySensitive captureDiagnostic recording/measuringSensorsMechanical HyperalgesiaEngineering
The invention relates to a portable mechanical hyperalgesia measuring apparatus. The portable mechanical hyperalgesia measuring apparatus is structurally characterized in that a probe is connected with a shaft lever, the shaft lever penetrates a barrel to be connected with a recording component, the recording component is connected with a pen refill, and a cambered-surface graduated scale is arranged beside the pen refill; a spring is arranged in the barrel, and a circular tube is connected onto the inner bottom of the barrel; the probe comprises a probe body and a base, a core comprises the shaft lever, a disc and an upper screw, the recording component comprises a metal wire, a metal tube and a lower screw, and a barrel component comprises the barrel, the cambered-surface graduated scale and the circular tube. The portable mechanical hyperalgesia measuring apparatus has the advantages that shortcomings of high prices, vulnerable probes, dependence on power sources, bulkiness and the like can be overcome by the aid of the portable mechanical hyperalgesia measuring apparatus; the portable mechanical hyperalgesia measuring apparatus is easy to manufacture, the probe, the shaft lever, the metal wire, the barrel, the circular tube, the spring and the like are frequently used articles, hyperalgesia degrees of four limbs of a rat can be measured by the portable mechanical hyperalgesia measuring apparatus, the portable mechanical hyperalgesia measuring apparatus is good in sensitivity and easy to operate and is energy-saving and environmental friendly as compared with the traditional method, and special devices, power sources and other special conditions can be omitted.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
Compositions and method for enhancing the therapeutic activity of opioids in treatment of pain
Compositions and methods for inhibiting opioid tolerance and opioid withdrawal-induced hyperalgesia are provided.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Antagonists to the vanilloid receptor subtype 1 (VR1) and uses thereof
Compounds having formula (I)or a pharmaceutically acceptable salt, prodrug, or salt of a prodrug thereof, wherein L, A, G, R1, R2 and R3 are as defined herein. These compounds are particularly useful in the treatment of pain, inflammatory hyperalgesia, and urinary dysfunctions, such as bladder overactivity and urinary incontinence.
Owner:ABBVIE INC
Use Of 3-Substituted-2-(Diphenylmethy)-1-Azabicyclo[2.2.2]Octanes For Treating Mrg-X1 Receptor Mediated Diseases
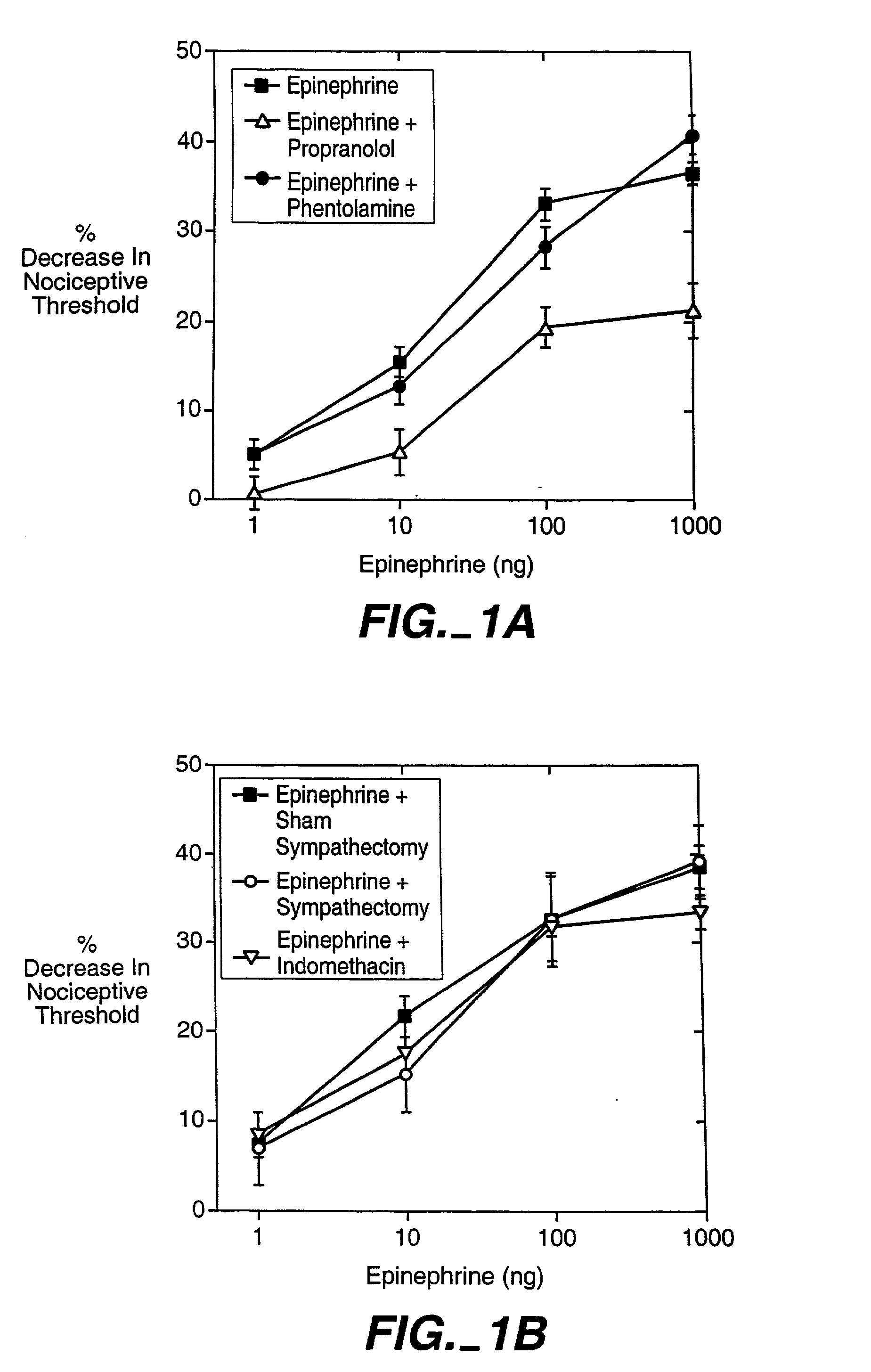
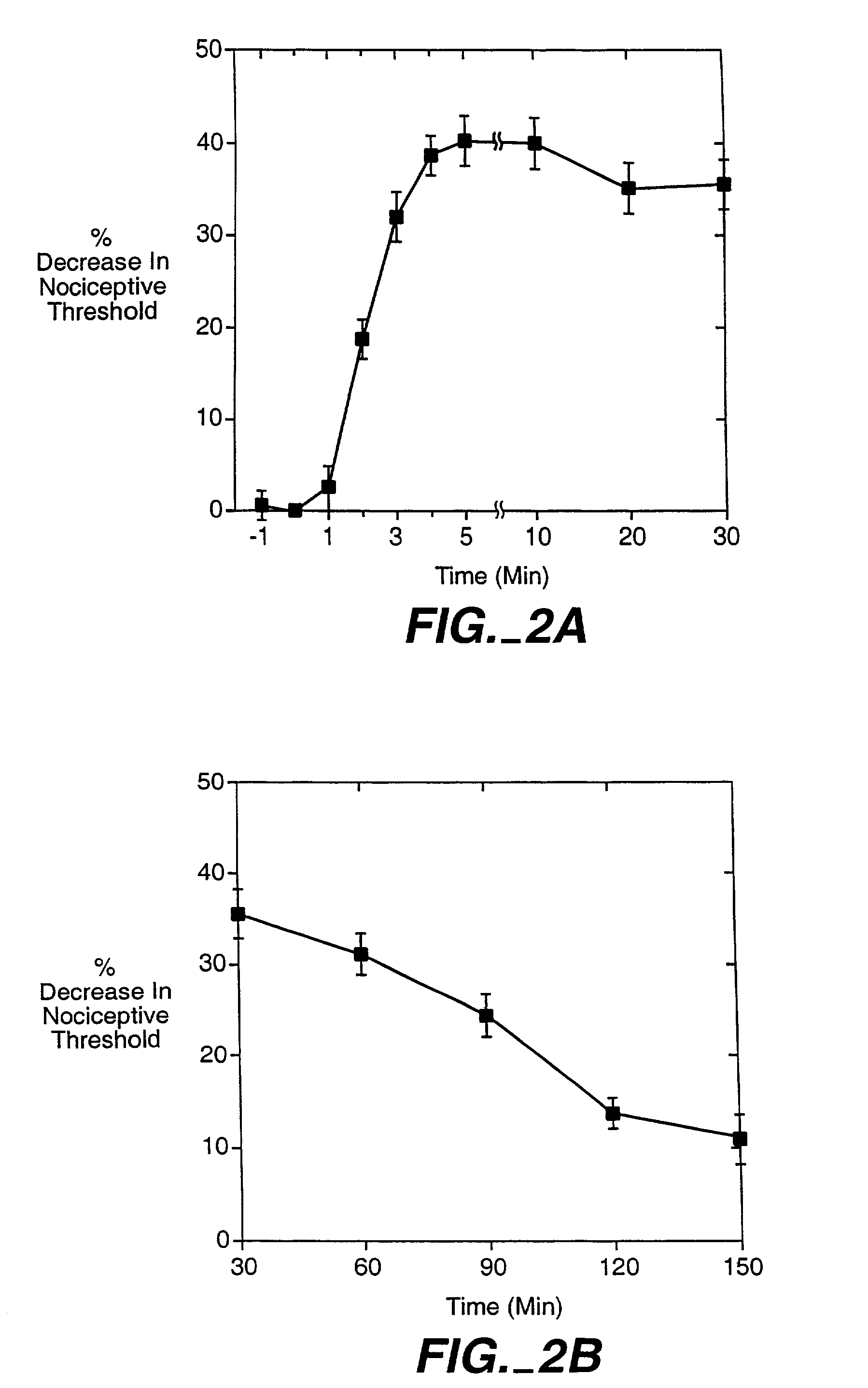
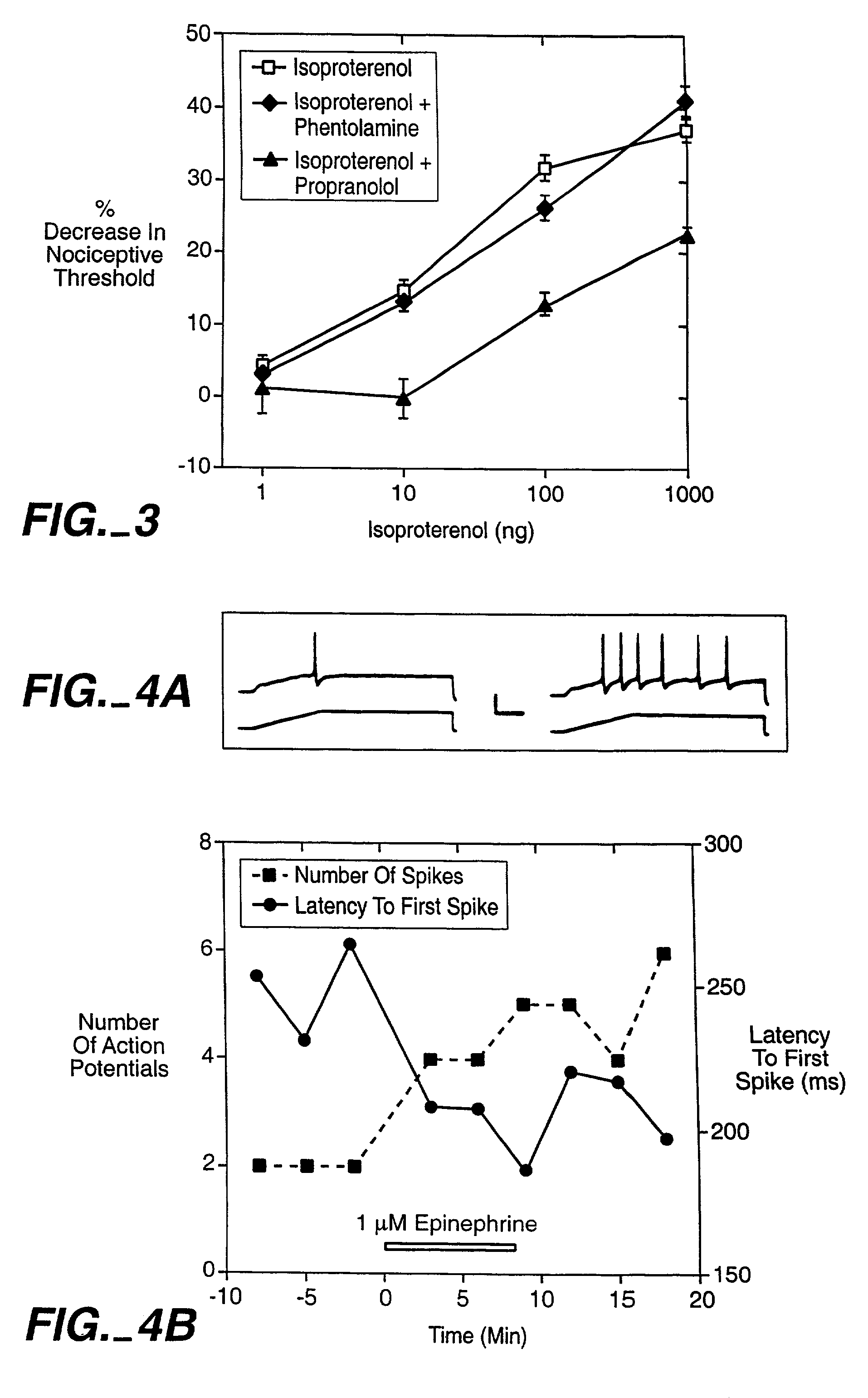
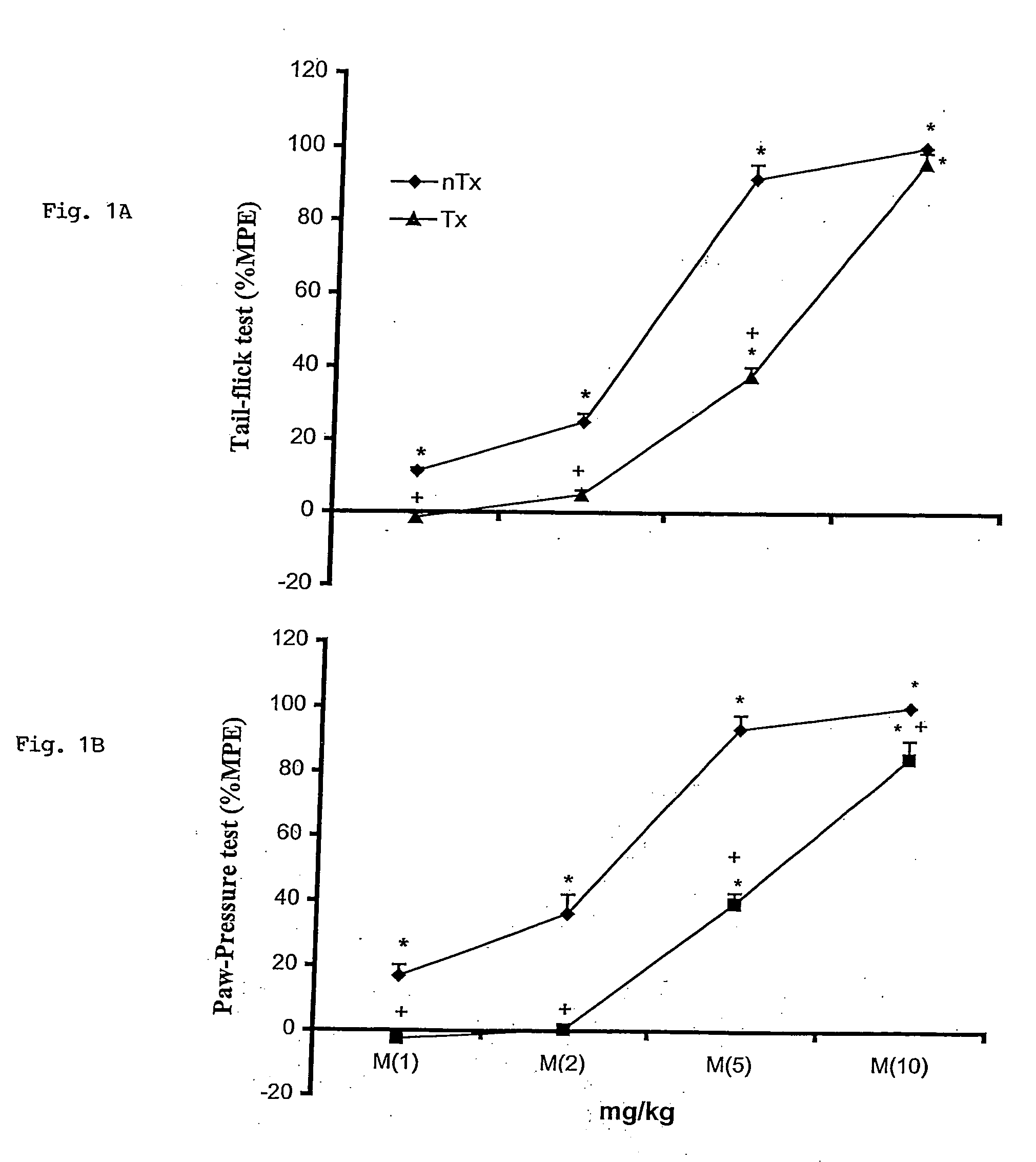
The invention encompasses a method for treating a disease or condition mediated by the human MRG-X1 receptor, such as nociception, hyperalgesia, allodynia, pain related to central hypersensitivity conditions, somatic pain, visceral pain, acute pain, chronic pain, post-operative pain, headache, inflammatory pain, neurological pain, musculoskeletal pain, cancer related pain or vascular pain, in a human patient in need thereof comprising administering to the patient a therapeutically effective amount of a 3-substituted-2-(diphenylmethy)-1-azabicyclo[2.2.2]octane or a pharmaceutically acceptable salt thereof. The invention is also directed to the use of these compounds as molecular tools to directly explore the role of the MRG-X1 receptor in pain perception.
Owner:MERCK SHARP & DOHME CORP
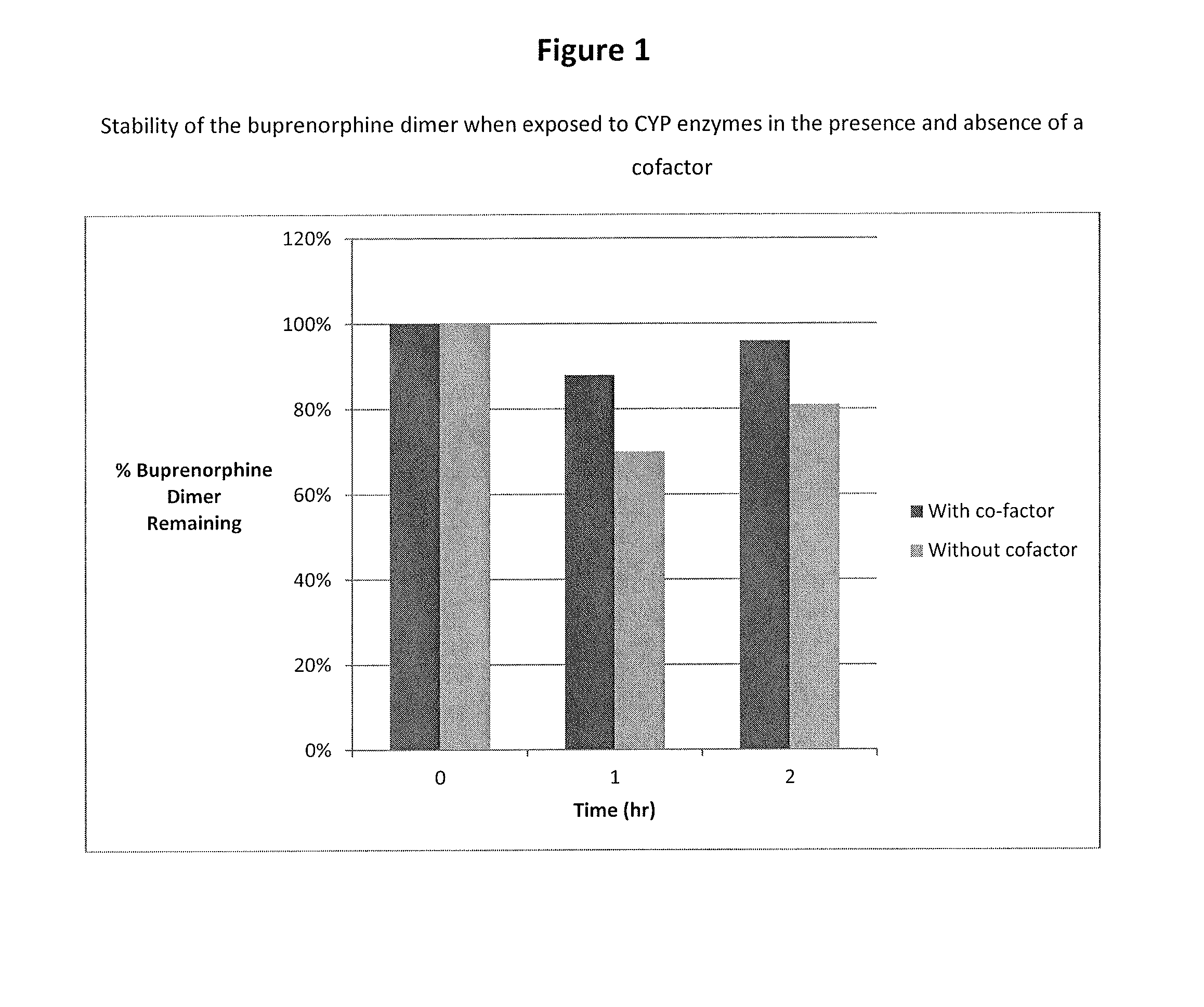
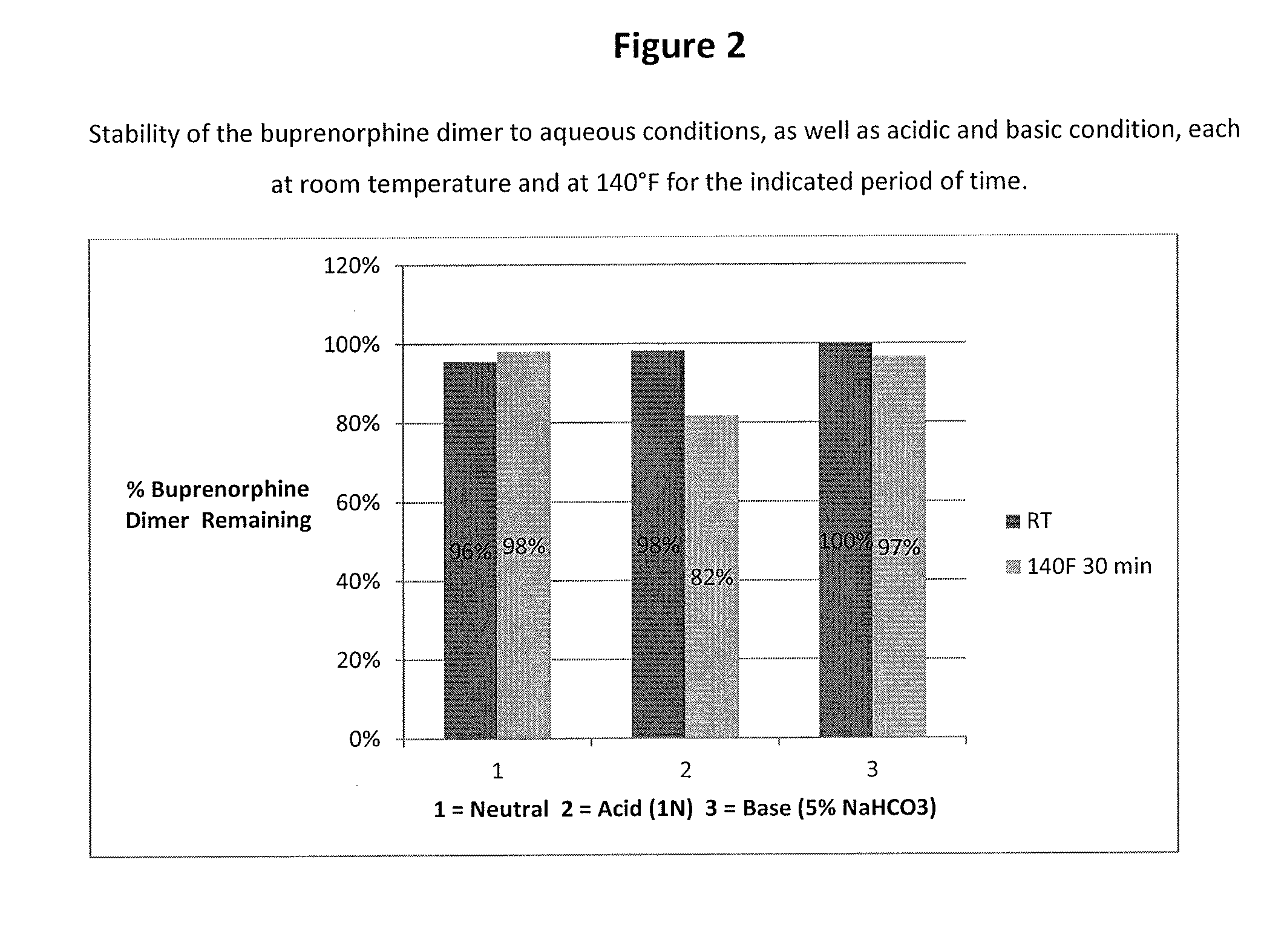
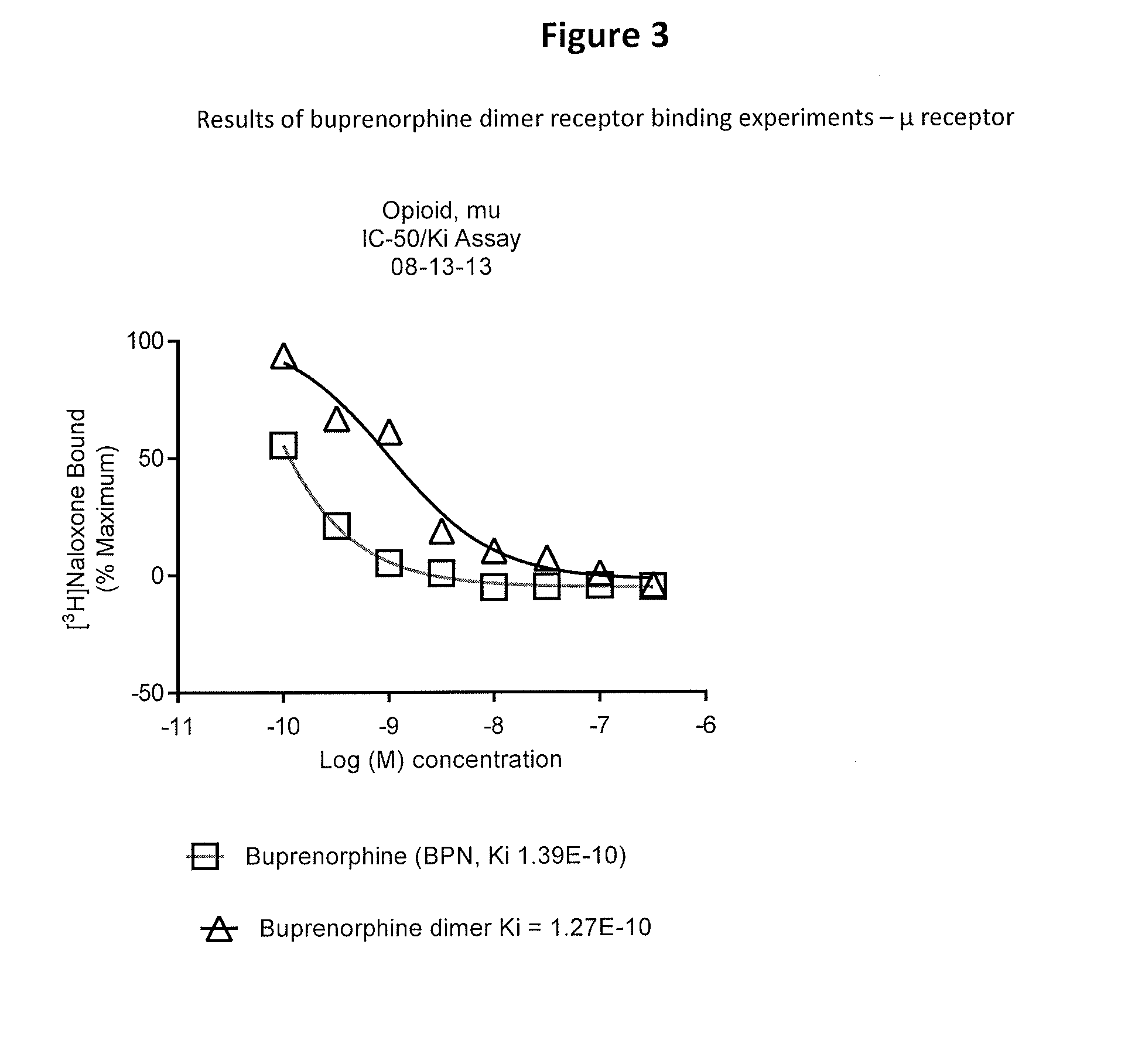
Buprenorphine dimer and its use in treatment of gastrointestinal disorders
ActiveUS20150307504A1Reduces visceral (colonic) hypersensitivityEffective treatmentBiocideNervous disorderDiseaseMedicine
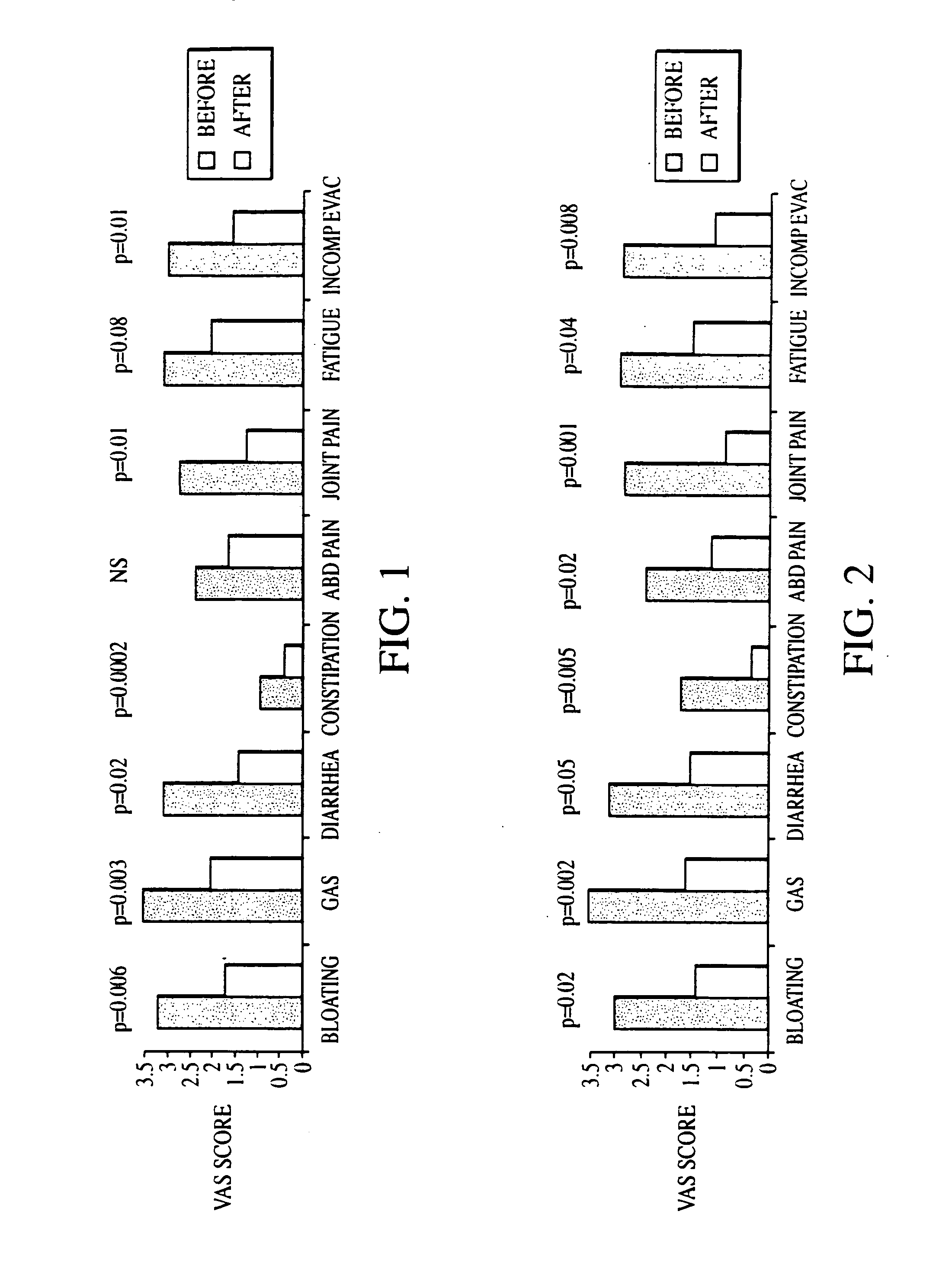
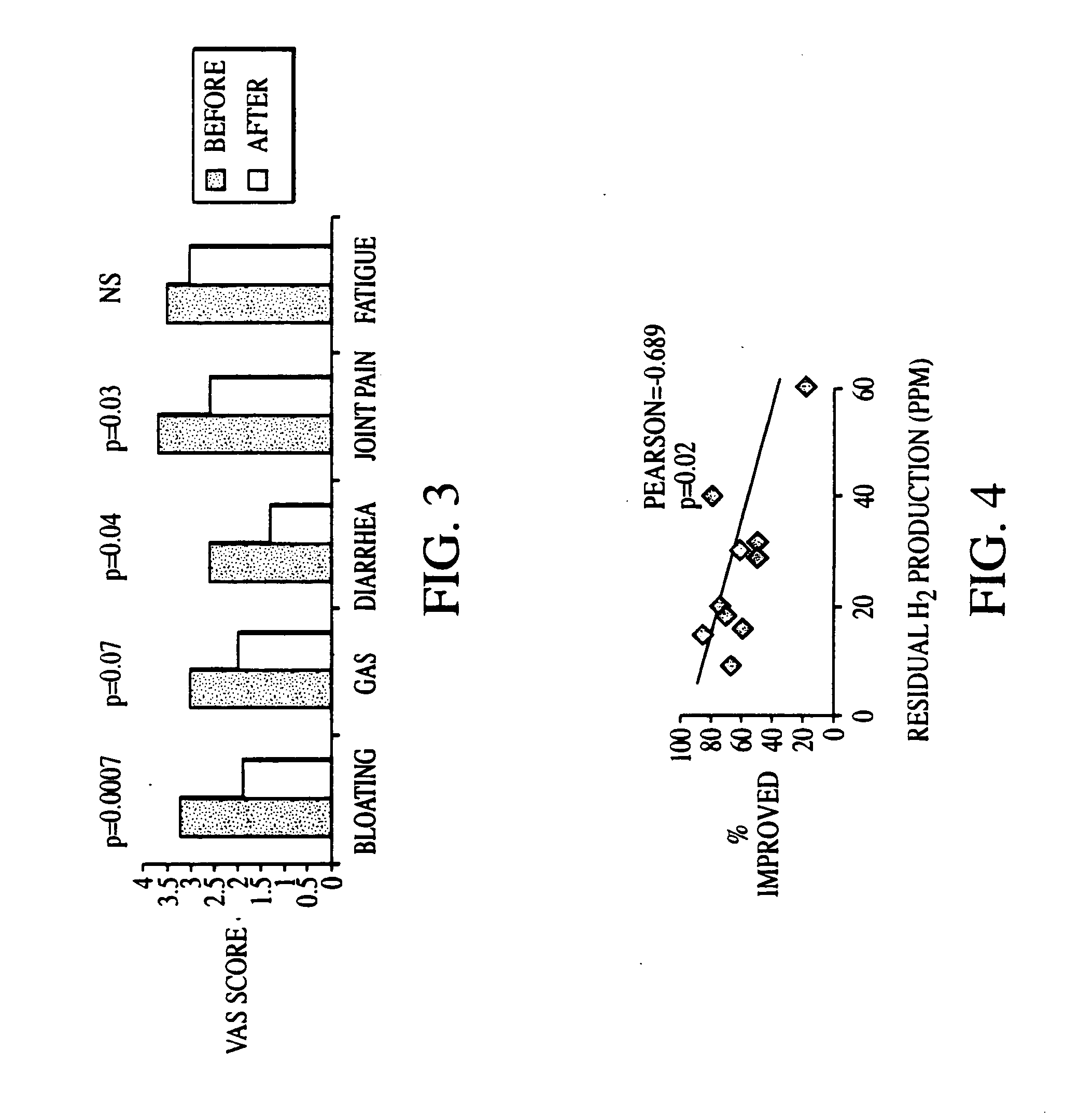
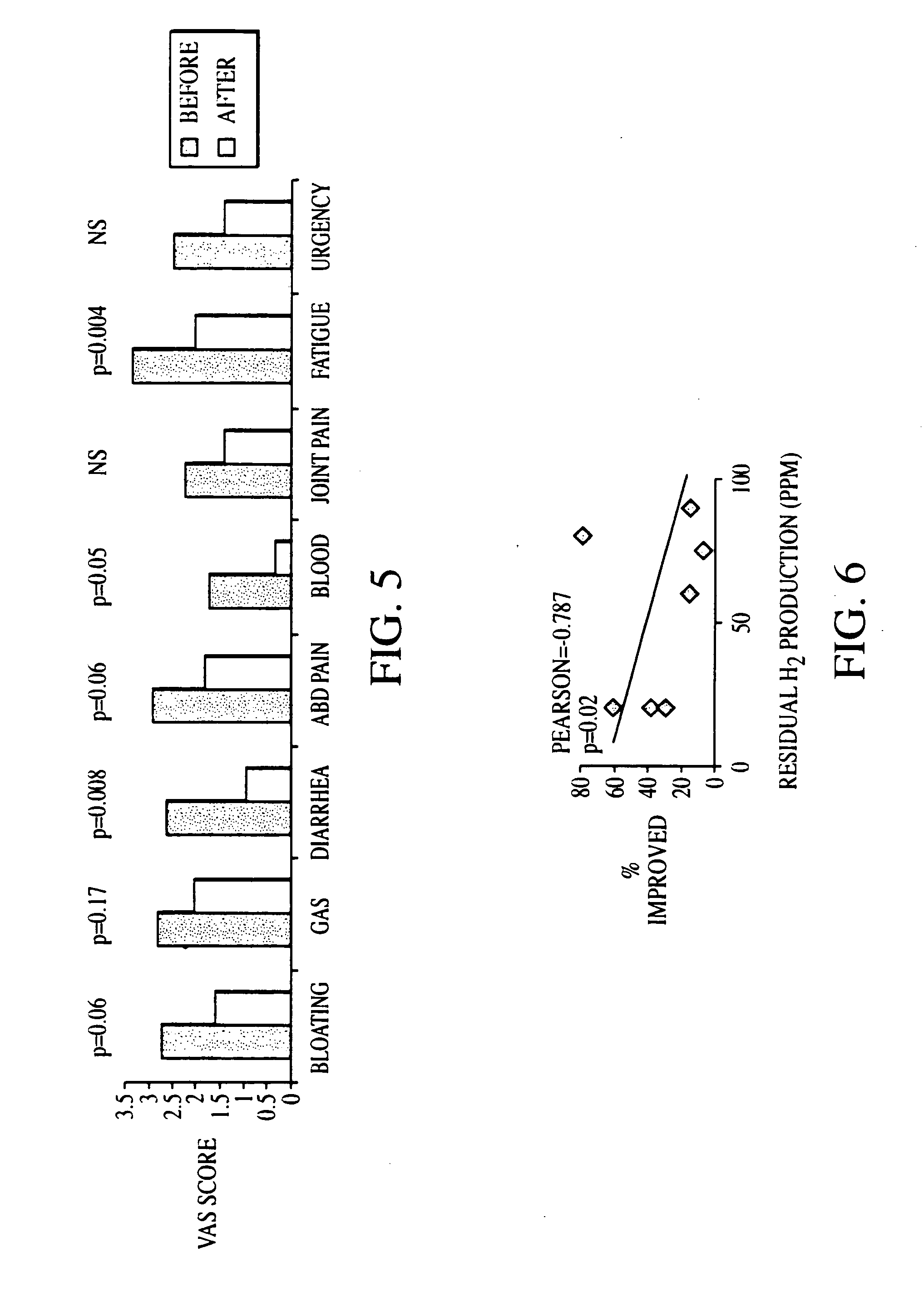
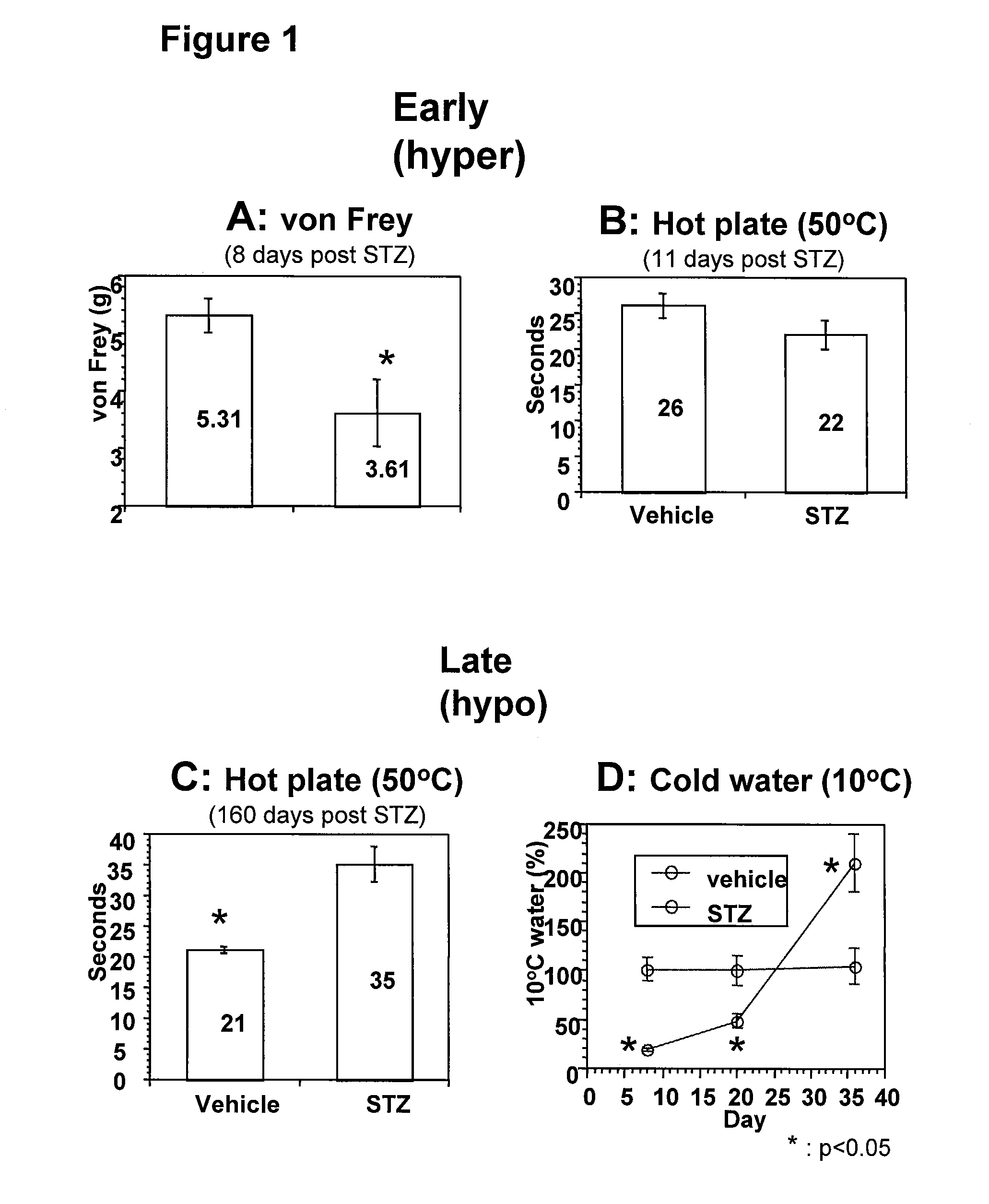
The present invention provides a buprenorphine dimer compound, wherein the two buprenorphine portions are linked via an ethylene spacer, wherein the spacer is bonded to the two opioid molecules via an ether bond. Pharmaceutical compositions comprising such a buprenorphine dimer drug are also disclosed, and the use of such compounds in the treatment of gastrointestinal hyperalgesia generally and in particular diarrhea-predominant irritable bowel syndrome.
Owner:DIMERX INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Use Of 3-Substituted-2-(Diphenylmethy)-1-Azabicyclo[2.2.2]Octanes For Treating Mrg-X1 Receptor Mediated Diseases Use Of 3-Substituted-2-(Diphenylmethy)-1-Azabicyclo[2.2.2]Octanes For Treating Mrg-X1 Receptor Mediated Diseases](https://images-eureka.patsnap.com/patent_img/894fab05-94a2-4585-ab84-f88256353dfb/US20080027095A1-20080131-D00000.png)
![Use Of 3-Substituted-2-(Diphenylmethy)-1-Azabicyclo[2.2.2]Octanes For Treating Mrg-X1 Receptor Mediated Diseases Use Of 3-Substituted-2-(Diphenylmethy)-1-Azabicyclo[2.2.2]Octanes For Treating Mrg-X1 Receptor Mediated Diseases](https://images-eureka.patsnap.com/patent_img/894fab05-94a2-4585-ab84-f88256353dfb/US20080027095A1-20080131-D00001.png)
![Use Of 3-Substituted-2-(Diphenylmethy)-1-Azabicyclo[2.2.2]Octanes For Treating Mrg-X1 Receptor Mediated Diseases Use Of 3-Substituted-2-(Diphenylmethy)-1-Azabicyclo[2.2.2]Octanes For Treating Mrg-X1 Receptor Mediated Diseases](https://images-eureka.patsnap.com/patent_img/894fab05-94a2-4585-ab84-f88256353dfb/US20080027095A1-20080131-D00002.png)