Norfloxacin and vanillin eutectic and preparation method thereof
A technology of norfloxacin and vanillin, which is applied in the field of new norfloxacin co-crystal and its preparation, and can solve problems such as limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
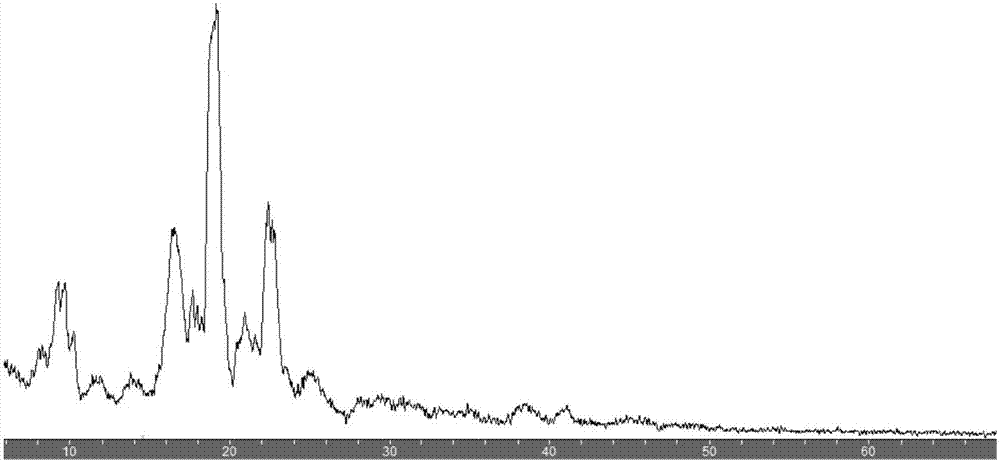
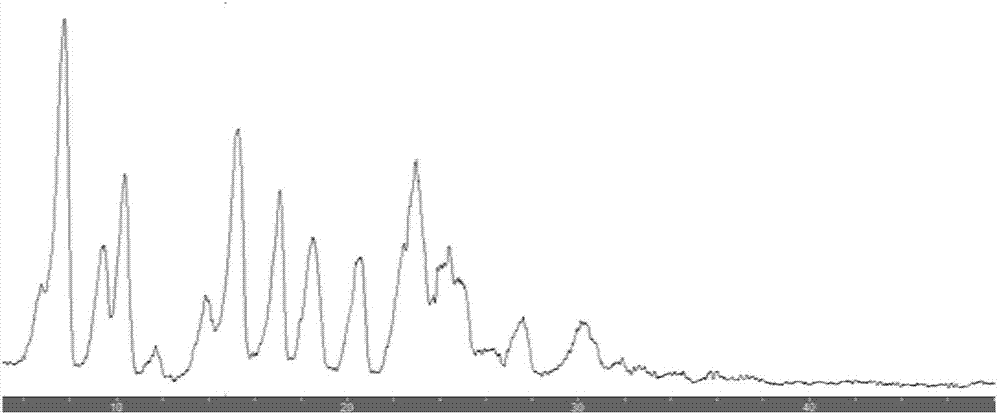
Image
Examples
specific Embodiment 1
[0024] Add 104g of deionized water into a 250ml three-neck flask, slowly add 348mg of norfloxacin and 166mg of vanillin, raise the temperature to 100°C, dissolve the norfloxacin and vanillin, filter the reaction solution quickly after dissolution, and place the filtrate in a 250ml beaker , naturally cooled, placed for 24 hours, crystals were precipitated, filtered, and dried to obtain 502 mg of needle-shaped white crystals, that is, norfloxacin and vanillin eutectic. Record the solubility of norfloxacin, norfloxacin and vanillin eutectic at 25 ℃ of pure water species as shown in table 1, as can be seen from the table, eutectic of the present invention compares with norfloxacin, greatly improves The solubility in water is improved, and the bioavailability is greatly improved; and it is also beneficial to the processing operation of the subsequent pharmaceutical process.
[0025] Table 1 Solubility of norfloxacin, norfloxacin and vanillin eutectic in pure water at 25°C
[0026]...
specific Embodiment 2
[0028] Add 174g of deionized water to a 250ml three-necked flask, slowly add 348mg of norfloxacin and 166mg of vanillin, raise the temperature to 90°C, dissolve the norfloxacin and vanillin, filter the reaction solution quickly after dissolution, and place the filtrate in a 250ml beaker , naturally cooled, placed for 24 hours, crystals were precipitated, filtered, and dried to obtain 502 mg of needle-shaped white crystals, that is, norfloxacin and vanillin eutectic. The measured solubility in eutectic water is shown in Table 2.
[0029] Table 2 Solubility of norfloxacin, norfloxacin and vanillin eutectic in pure water at 25°C
[0030] compound Solubility (mg / mL) Norfloxacin 0.28 Co-crystal of norfloxacin and vanillin 27.9
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com