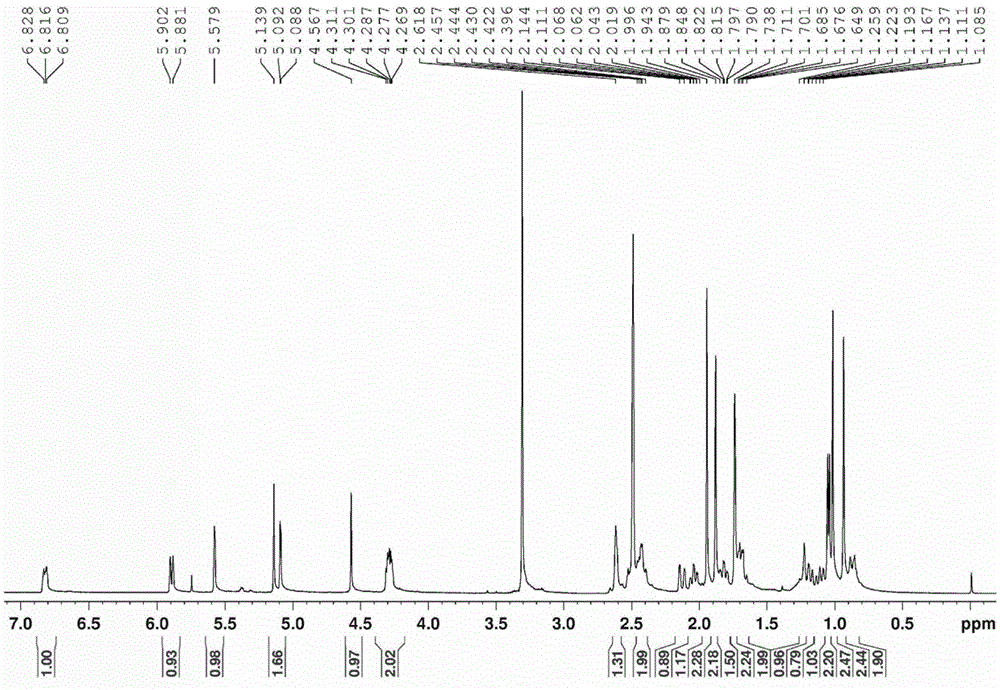
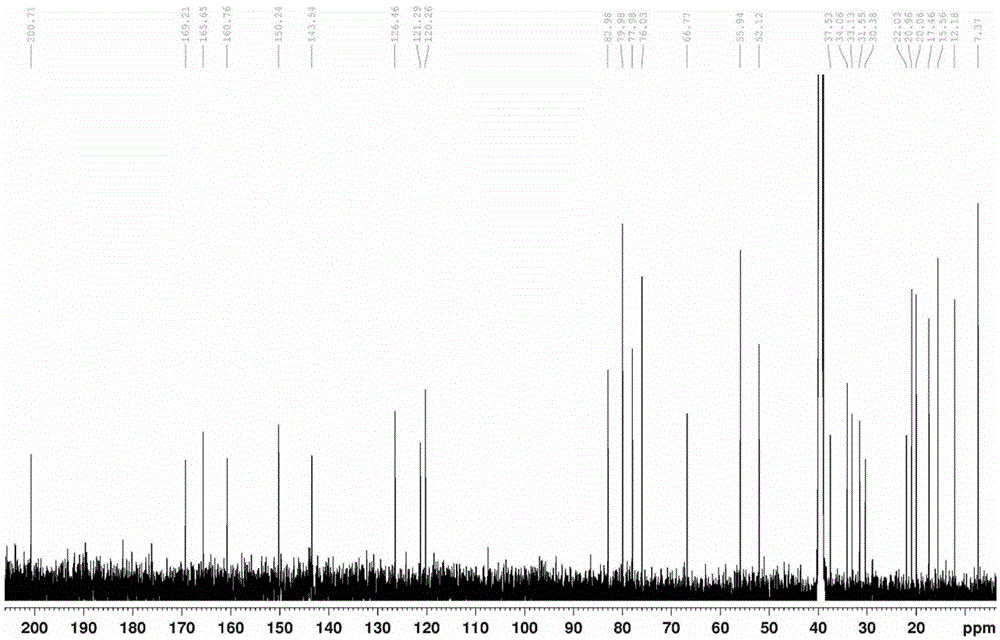
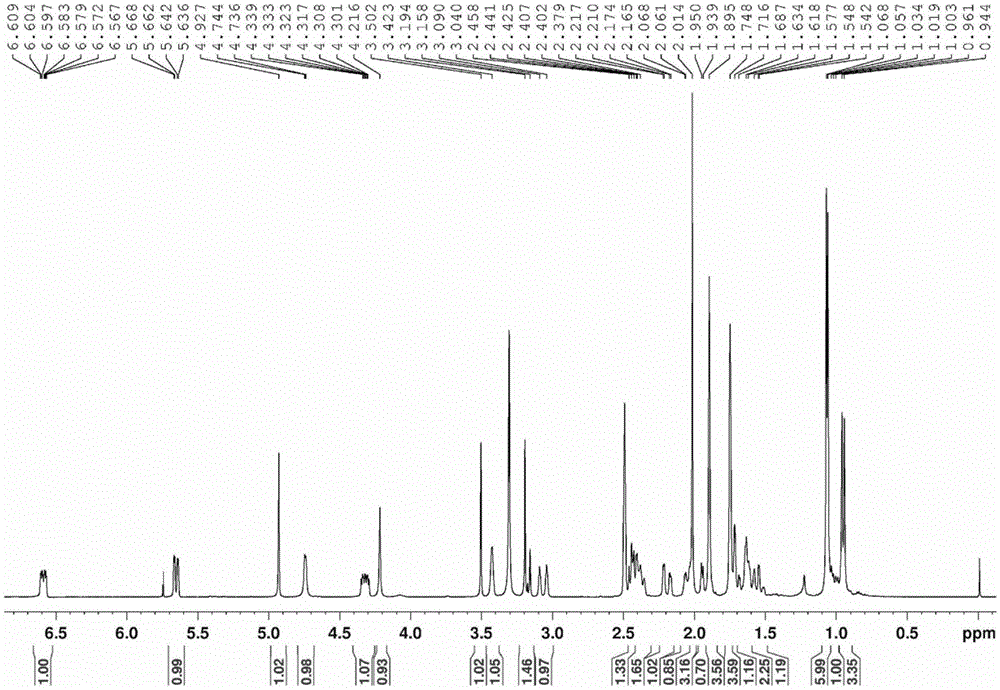
Compound having quinone reductase induced activity and preparation method thereof
A technology for inducing activity and reductase, applied in the fields of steroids, organic chemistry, drug combination, etc., which can solve the problems of no artificial citation cultivation and few reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1) Put 1Kg of the persistent calyx into the extraction tank, add an ethanol aqueous solution containing 95% ethanol by volume (that is, 5Kg of the ethanol aqueous solution) that is 5 times the weight of the persistent calyx, and extract under reflux at 90°C. The time of each extraction is 2h, and the total extraction is 4 times (a total of 20Kg in ethanol aqueous solution), and the extraction liquid is combined;
[0057] 2) Concentrate the extract obtained in step 1) (recover ethanol) to nearly dryness to obtain 130 g of thick extract, dilute with 1 L of water, extract with 1 L of ethyl acetate each time, extract 3 times in total, and combine the extracts;
[0058] 3) 20 g of the extract obtained by concentrating the extract obtained in step 2) is passed through a silica gel chromatographic column (wet column packing, dry loading), wherein the filler used in the silica gel chromatographic column is 100 mesh column chromatography silica gel , the weight ratio of extract ...
Embodiment 2
[0067] 1) Put 1Kg of the persistent calyx in the extracting tank, add 3 times the weight of the persistent calyx in each time an ethanol aqueous solution containing 95% ethanol by volume (that is, 3Kg of the ethanol aqueous solution), and reflux extraction at 100°C. The time of each extraction is 3h, and the total extraction is 2 times (the ethanol aqueous solution is 6Kg in total), and the extracts are combined;
[0068] 2) Concentrate the extract obtained in step 1) (recover ethanol) to nearly dryness to obtain 110 g of thick extract, dilute with 1 L of water, extract with 1 L of ethyl acetate each time, extract 3 times in total, and combine the extracts;
[0069] 3) 16g of extract obtained by concentrating the extract obtained in step 2) is passed through a silica gel chromatographic column (wet column packing, dry loading), wherein the filler used in the silica gel chromatographic column is 200 mesh column chromatography silica gel , the weight ratio of extract to column c...
Embodiment 3
[0072] Embodiment 3 (quinone reductase induction experiment)
[0073] Take logarithmic phase mouse liver cancer cells (Hepa1c1c7, purchased from ATCC), 1×10 per well 4Inoculate on a 96-well plate, add 200 μL of DMEM medium to each well, discard the supernatant after 12 hours, and then add compound A (0.106 mg) with quinone reductase activity prepared in Example 1 to three duplicate wells, and another Select 3 multiple wells to add compound B with cancer prevention effect prepared in Example 1, and finally add compound C with cancer prevention effect prepared in Example 1 to the other 3 multiple wells, corresponding to the compound dosing group A. Compound dosing group B and compound dosing group C; add 4′-bromoflavone (0.106 mg) to 3 duplicate wells, which is the positive control dosing group; add dimethyl sulfoxide to 3 duplicate wells (0.106mg), which is the negative control group; the blank group is 200μL DMEM medium without adding drugs. After culturing for 24 h, the sup...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com