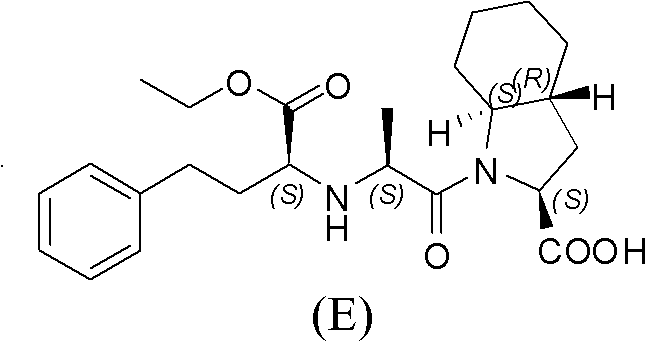
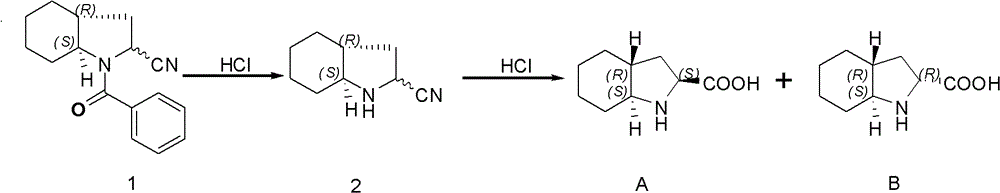
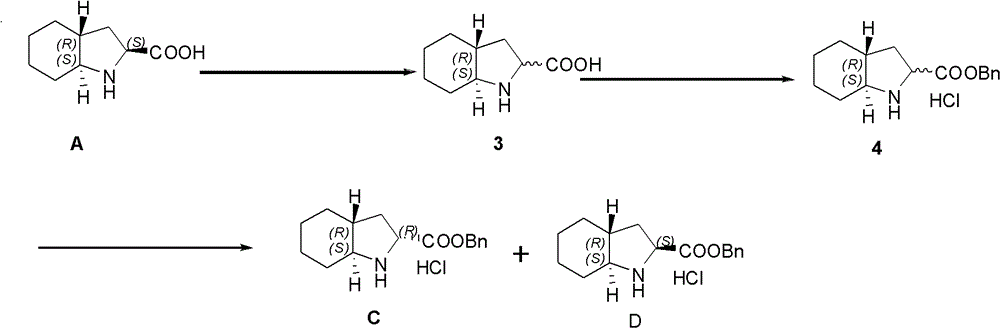
The preparation method of the isomer of trandolapril intermediate
A technology for trandolapril and intermediates, applied in the field of isomer preparation, can solve problems such as difficulty in obtaining, preparation and separation of compounds, and influence on the establishment of the quality system of trandolapril, achieving easy realization and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1. Preparation of Isomer Mixture 3
[0030] 10 g of Compound A was added dropwise to pH=5.8 with concentrated ammonia water, and freeze-dried until no ice appeared on the round bottom flask. The obtained solid, 7.29g (2S, 3S)-tartaric acid, 1.2mL n-butyraldehyde, and 146mL n-butyric acid were successively added into a three-necked flask, and the temperature was raised to 80°C. After 18h, the reaction was stopped and cooled in an ice-water bath. After filtering, the filter cake was washed with cold ether and dried to obtain tartaric acid complex. The complex was poured into a 1000mL reaction flask, and 350mL of methanol was added; concentrated ammonia water was added dropwise in an ice-water bath to adjust the pH to 9, and stirring was continued for 30mins. Suction filtration, and the filtrate was spin-dried to obtain a khaki solid. Add 60mL of methanol, heat to dissolve, and dropwise add methyl tert-butyl ether to the system until no solids are produced, filte...
Embodiment 2
[0031] Embodiment 2. Preparation of Compound C
[0032] Add 3.8mL of benzyl alcohol into a 25mL reaction flask, cool down to -10°C, add 0.845g of the isomer mixture 3 obtained in the previous step to the reaction flask, add 1.6mL of thionyl chloride dropwise into the reaction flask, and add dropwise During the process, gas is produced. After the dropwise addition, it was raised to room temperature and reacted for 16h. Add 5 mL of anhydrous diethyl ether to the system and stir for 2 h. After filtration, the filtrate was rotary evaporated until no liquid dripped out, then diethyl ether was added and stirred until 0.49 g of a white solid was precipitated. The preparation was separated to obtain product C, 0.19 g of (R, S, R)-benzyl octahydroindole-2-carboxylate hydrochloride.
[0033] (R, S, R)-benzyl octahydroindole-2-carboxylate hydrochloride:
[0034] MS (m / z): 260 (Base+H + ), 282 (Base+Na + ).
[0035] 1 H NMR (400MHz, Acetone-d 6 ): δ=7.28-7.45(m, 5H), 5.18(q, J=7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com