Crystal form B of lurasidone hydrochloride and preparation method thereof
A technique for the crystal form of alkanediimide hydrochloride, which is applied to the crystal form B of lurasidone hydrochloride and its preparation field, and can solve problems such as crystal forms of compounds not mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
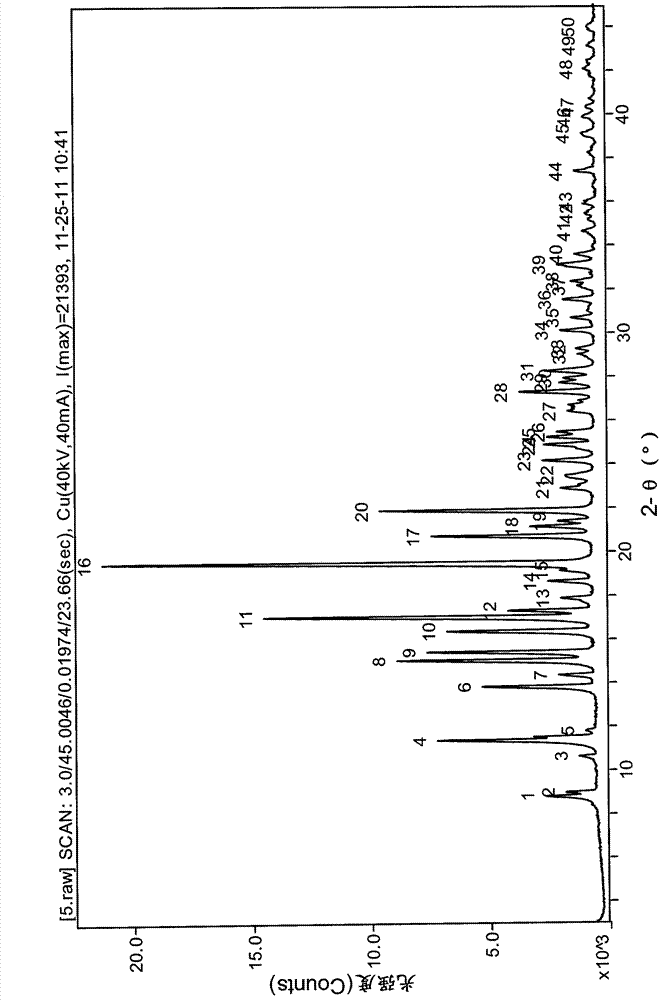
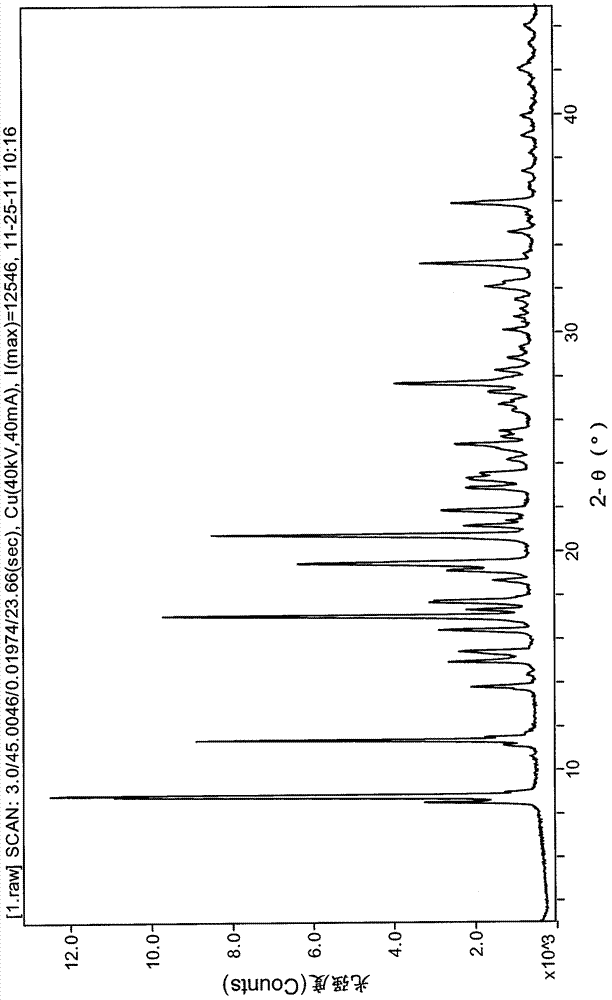
[0029] 10g N-[4-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-(2R,3R)-2,3-tetramethylene-butyl]- (1'R, 2'S, 3'R, 4'S)-2,3-bicyclo[2,2,1]heptane diimide hydrochloride was added to a mixed solution of 100mL acetone and 36mL water, heated to 40 ℃ dissolve clear, cooling. The obtained solution was left to stand, and the solid was precipitated by filtration, and dried to obtain 5.9 g of a white solid. The obtained product was identified as Form B by X-ray powder diffraction.
Embodiment 2
[0031] 10g N-[4-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-(2R,3R)-2,3-tetramethylene-butyl]- Add (1'R, 2'S, 3'R, 4'S)-2,3-bicyclo[2,2,1]heptanediimide hydrochloride into 300mL acetone, heat to reflux until dissolved, and continue heating for 1h ,cool down. The resulting solution was stirred overnight, filtered, and dried to obtain 6.8 g of a white solid. The obtained product was identified as Form B by X-ray powder diffraction.
Embodiment 3
[0033] 10g N-[4-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-(2R,3R)-2,3-tetramethylene-butyl]- Add (1'R, 2'S, 3'R, 4'S)-2,3-bicyclo[2,2,1]heptanediimide hydrochloride into 200mL methanol, heat to reflux until dissolved, and continue heating for 1h ,cool down. The resulting solution was stirred overnight, filtered, and dried to obtain 6.9 g of a white solid. The obtained product was identified as Form B by X-ray powder diffraction.
PUM
| Property | Measurement | Unit |
|---|---|---|
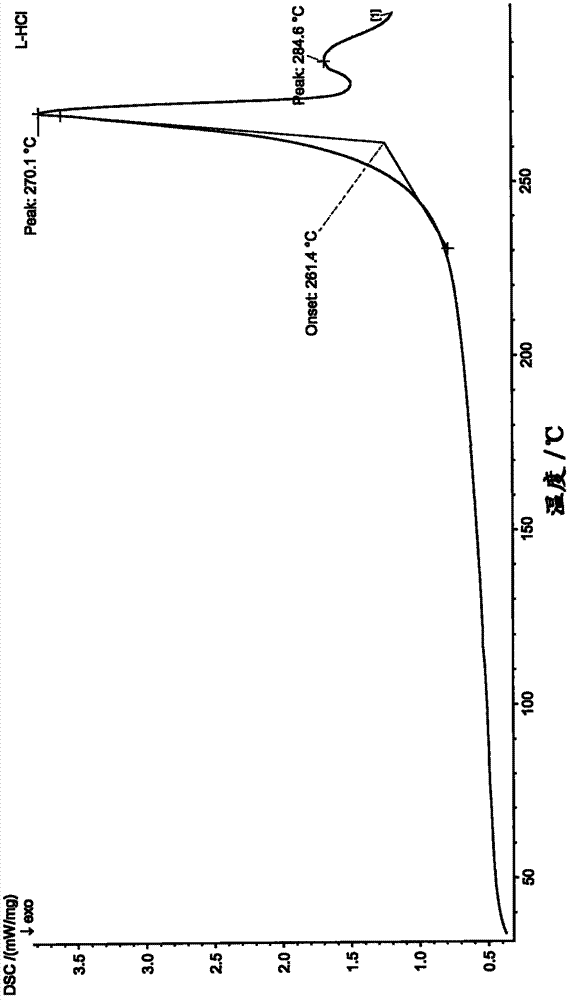
| decomposition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com