Synthesis method of 5-methyl-2-aminothiazole
The technology of an aminothiazole and a synthesis method, which is applied in the direction of organic chemistry and the like, can solve the problems of harsh preparation conditions of 2-chloropropionaldehyde, difficult separation and purification, and high requirements for production conditions, and achieves low cost of raw materials, stable intermediates, and high raw materials. easy-to-get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
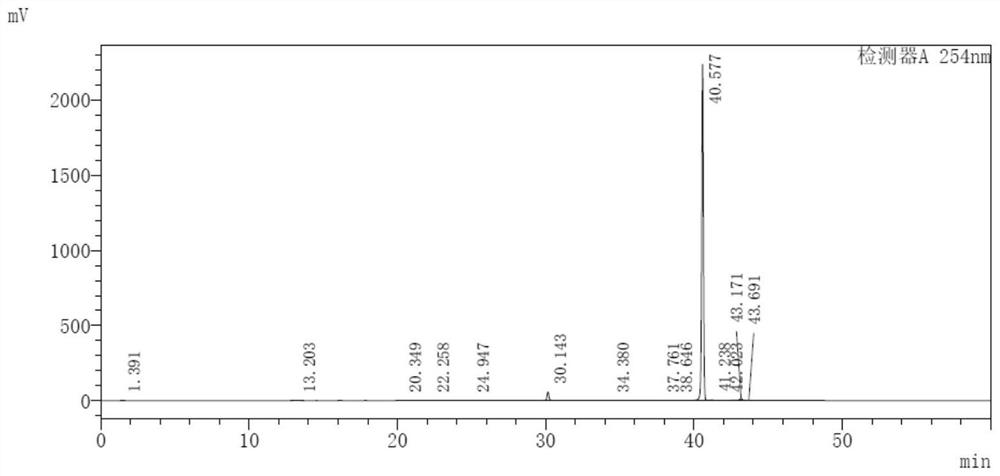
[0032] Dissolve 12.3g of methyl 2-chloropropionate and 8.0g of thiourea in 100mL of DMF, slowly heat to 80°C, keep for 5 hours, then cool down to room temperature, add 100mL of distilled water, a bright yellow precipitate precipitates, filter, and 20mL of ice water Wash the filter cake, place the filter cake in an oven, and dry it at 60° C. to constant weight to obtain 12.1 g of bright yellow powder 5-methyl-2-amino-4(5H)thiazolone (I), with a yield of 93%.
Embodiment 2
[0034] Dissolve 12.3g of methyl 2-chloropropionate and 8.0g of thiourea in 100mL of DMF, cool to 0°C, keep for 5 hours, add 100mL of distilled water, precipitate a bright yellow precipitate, filter, wash the filter cake with 20mL of ice water, and The filter cake was placed in an oven and dried at 60°C until constant weight to obtain 10.9 g of bright yellow powder with a yield of 83%.
Embodiment 3
[0036] Dissolve 12.3g of methyl 2-chloropropionate and 11.5g of thiourea in 100mL of DMF, slowly heat to 100°C, keep for 5 hours, then cool down to room temperature, add 100mL of distilled water, a bright yellow precipitate precipitates, filter, 20mL of ice water Wash the filter cake, place the filter cake in an oven, and dry at 60°C until constant weight to obtain 10.2 g of bright yellow powder 5-methyl-2-amino-4(5H)thiazolone (I), with a yield of 76.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com