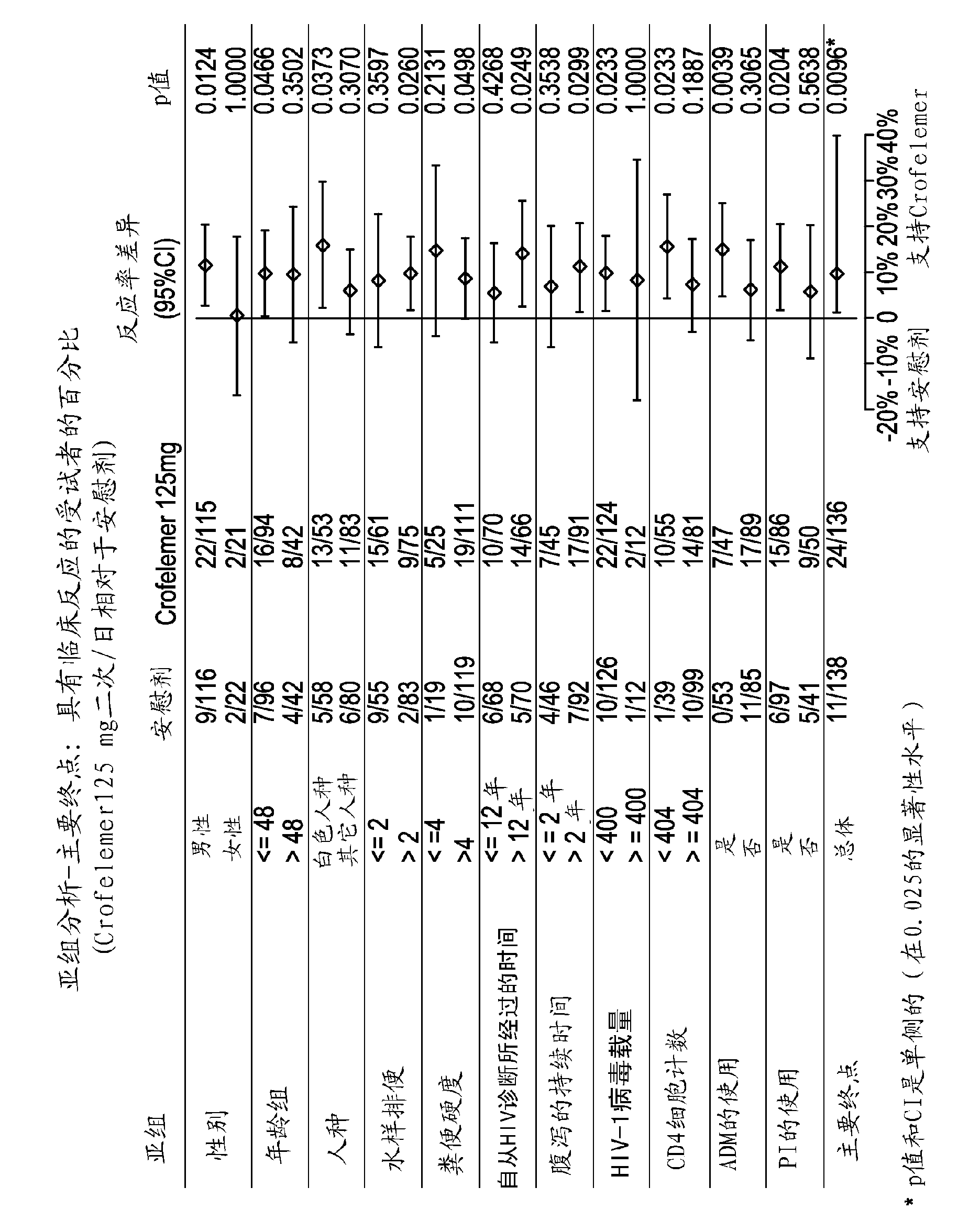
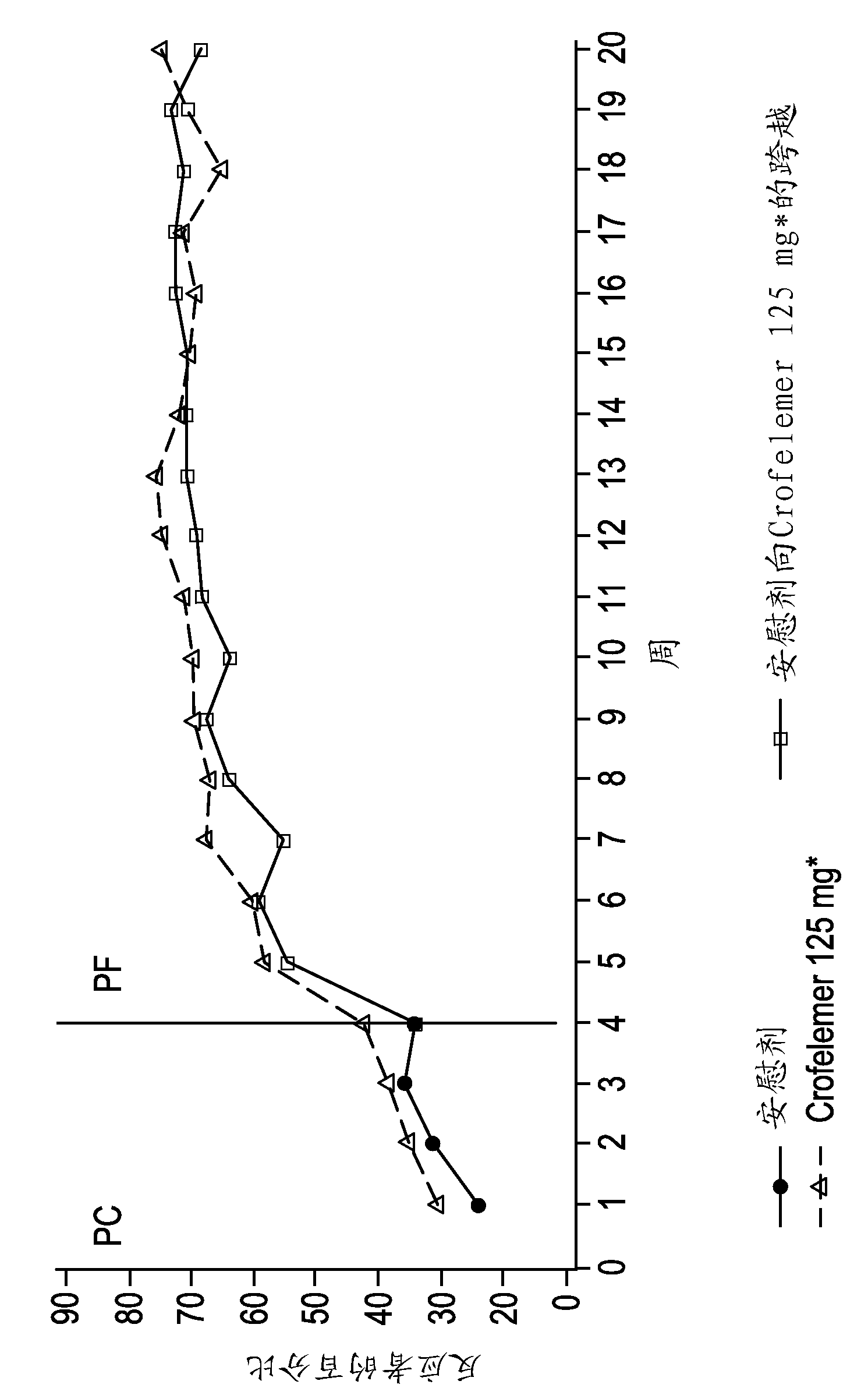
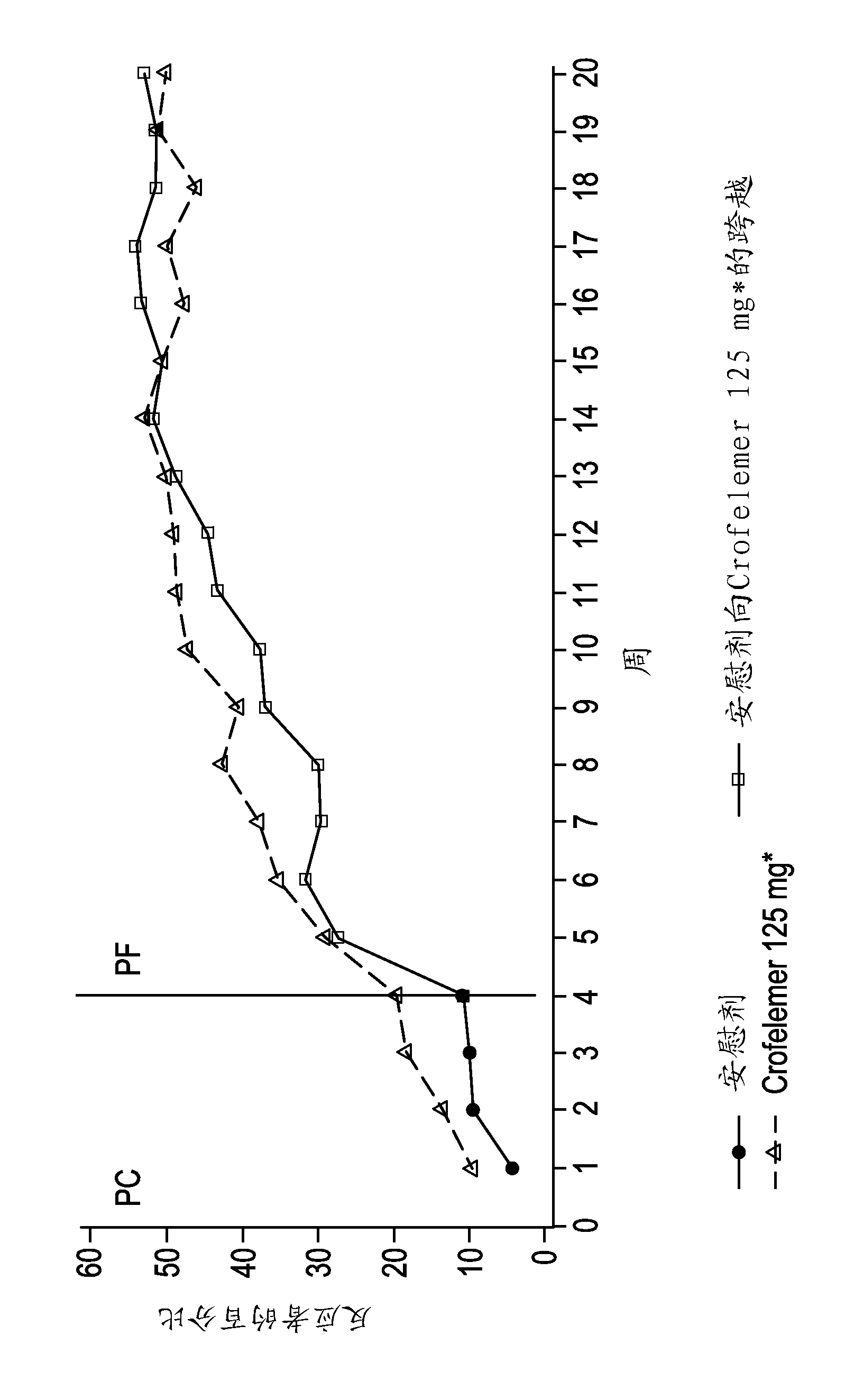
Methods and compositions for treating HIV-associated diarrhea
A correlation, diarrhea technology that can be used in drug combinations, active ingredients of hydroxyl compounds, pharmaceutical formulations, etc., to solve problems such as addiction possibility, unacceptable side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] Examples of the preparation and use of crofelemers are described in US Patent 7,556,831, US Patent Publication 20070254050, and US Patent Publication 20080031984, all of which are incorporated herein by reference in their entirety.
[0077] One embodiment includes a pharmaceutical composition comprising crofelemer and a pharmaceutically acceptable carrier.
[0078] The pharmaceutical composition described herein may further comprise excipients, such as one or more of diluents, binders, lubricants, disintegrants, coloring agents, flavoring agents or sweeteners. The composition can be formulated as coated and uncoated tablets of choice, hard and soft gelatin capsules, sugar-coated pills, lozenges, dry-paste tablets, pellets and powders in hermetically sealed packs. For example, the compositions may be formulated for topical use, such as ointments, pomades, creams, gels and lotions.
[0079] In certain embodiments, these pharmaceutical compositions are suitable for topica...
Embodiment 1
[0106] Example 1: Pulmonary Effects of Orally Administered Crofelemer in Rats
[0107] Crofelemer was administered to three treatment groups of 8 male rats at dose levels of 60, 200 and 600 mg / kg, respectively. Another group of 8 male rats was used as control animals, and vehicle (purified water) was administered. Crofelemer and vehicle were administered at a dose volume of 10 mL / kg. Another group of 8 male rats received the positive control baclofen at a dose level of 100 mg / kg and a dose volume of 15 mL / kg. All groups were dosed with crofelemer, positive control and vehicle by oral gavage.
[0108] For all animals, observations were made at least twice / day for mortality, morbidity, injury, and availability of food and water. Clinical observations were performed prior to dosing, approximately 1 hour after dosing, and after completion of the pulmonary monitoring period (approximately 4 hours after dosing). Body weight was measured and recorded prior to dosing (Day 1). Lun...
Embodiment 2
[0110] Example 2: 13-week oral toxicity study of Crofelemer administered to mice
[0111] Crofelemer was administered to three treatment groups of 15 male and 15 female mice at dose levels of 40, 400 and 1200 mg / kg / day, respectively. Another group of 15 animals / sex served as controls and received vehicle (purified water). Vehicle or crofelemer was administered to all groups at a dose volume of 10 mL / kg. Additionally, four groups of 8 or 39 animals / sex / group were used as toxicokinetic (TK) animals and dosed with 0, 40, 400 or 1200 mg / kg / day in the same manner as the main study group Dose levels received either control or crofelemer. Main study animals and TK animals were dosed with crofelemer at 1200 mg / kg / day for up to 55 or 56 days, respectively, due to death.
[0112] For all animals, secondary / daily observations were performed for morbidity, mortality, injury, and availability of food and water. Weekly clinical observations of detailed clinical signs were performed on a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com