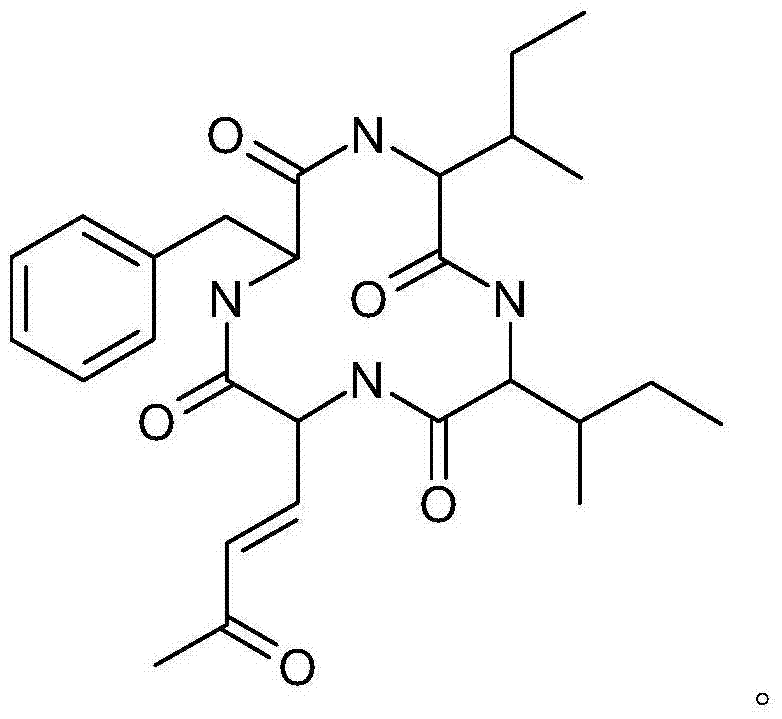
A compound having inhibitory activity against the glycine transporter
A technology for inhibiting activity and compounds, which is applied in the direction of drug combinations, chemical instruments and methods, and cyclic peptide components, and can solve problems such as the same structure of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
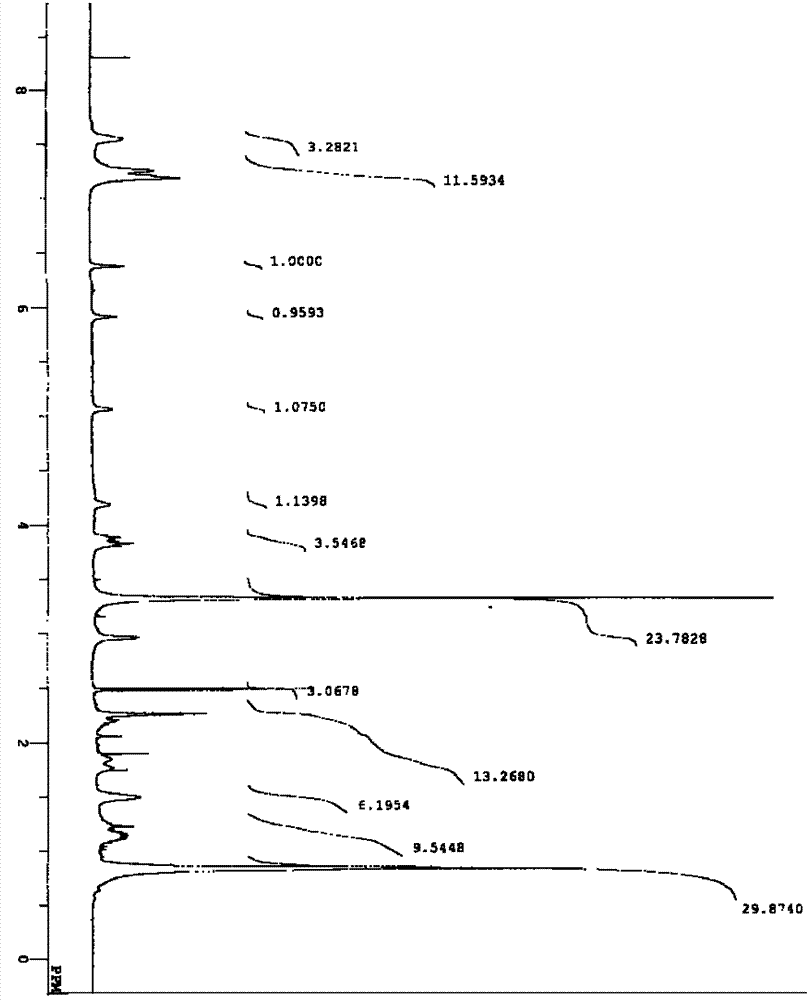
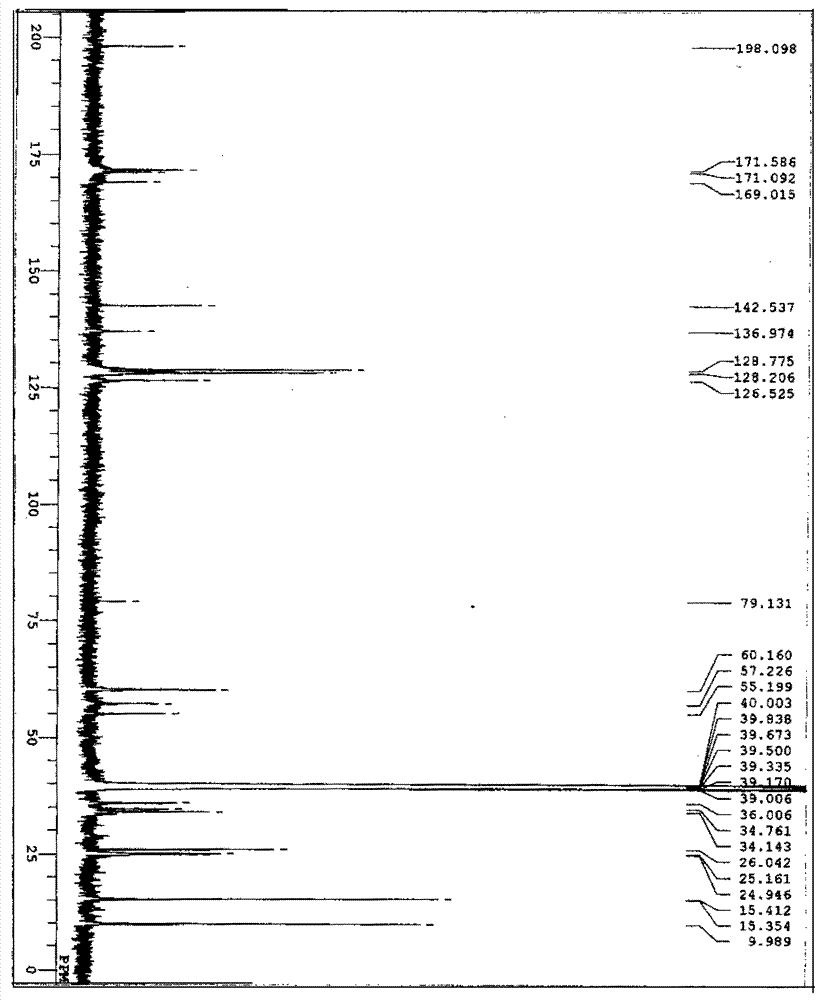
[0033] The preparation of embodiment 1 compound C6035G
[0034] 1. Adjust the pH of the liquid medium containing 1% glucose, 2.5% soluble starch, 0.3% cottonseed meal, 0.5% fish meal, 0.3% NZ CASE, 0.2% yeast extract and 0.2% calcium carbonate to 7.0, at 120°C Sterilize under moist heat for 20 minutes. Then inoculate an appropriate amount of strain CGMCC No.0792 under aseptic operation, shake culture at 28° C. and 180 rpm for 3 days, and use it as a seed culture solution.
[0035] Then add 1% glycerol, 0.5% glucose, 1% soluble starch, 0.1% (NH 4 ) 2 SO 4 ,
[0036] 0.05% MgSO 4 ·7H 2 O, 0.2% calcium carbonate and L-isoleucine 0.2% liquid culture medium 100ml adjust pH to 7.0 and pack in the 500ml Erlenmeyer shaker flask, prepare 100 bottles altogether, in 120 ℃, moist heat sterilization 20 minutes. Then, under aseptic operation, the above-mentioned seed culture solution was added thereto according to 10% inoculum amount, and cultured with shaking at 28° C. and 180 rpm f...
Embodiment 2
[0040] Example 2 Inhibitory Experiment on Rat Neuroglial Cells
[0041] 1. Specimen
[0042] Compound C6035G was dissolved in dimethyl sulfoxide and prepared to a concentration of 10 mg / ml, diluted with sterilized water to the target concentration for use.
[0043] 2. Experimental cells
[0044] Rat glial cells C6.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com