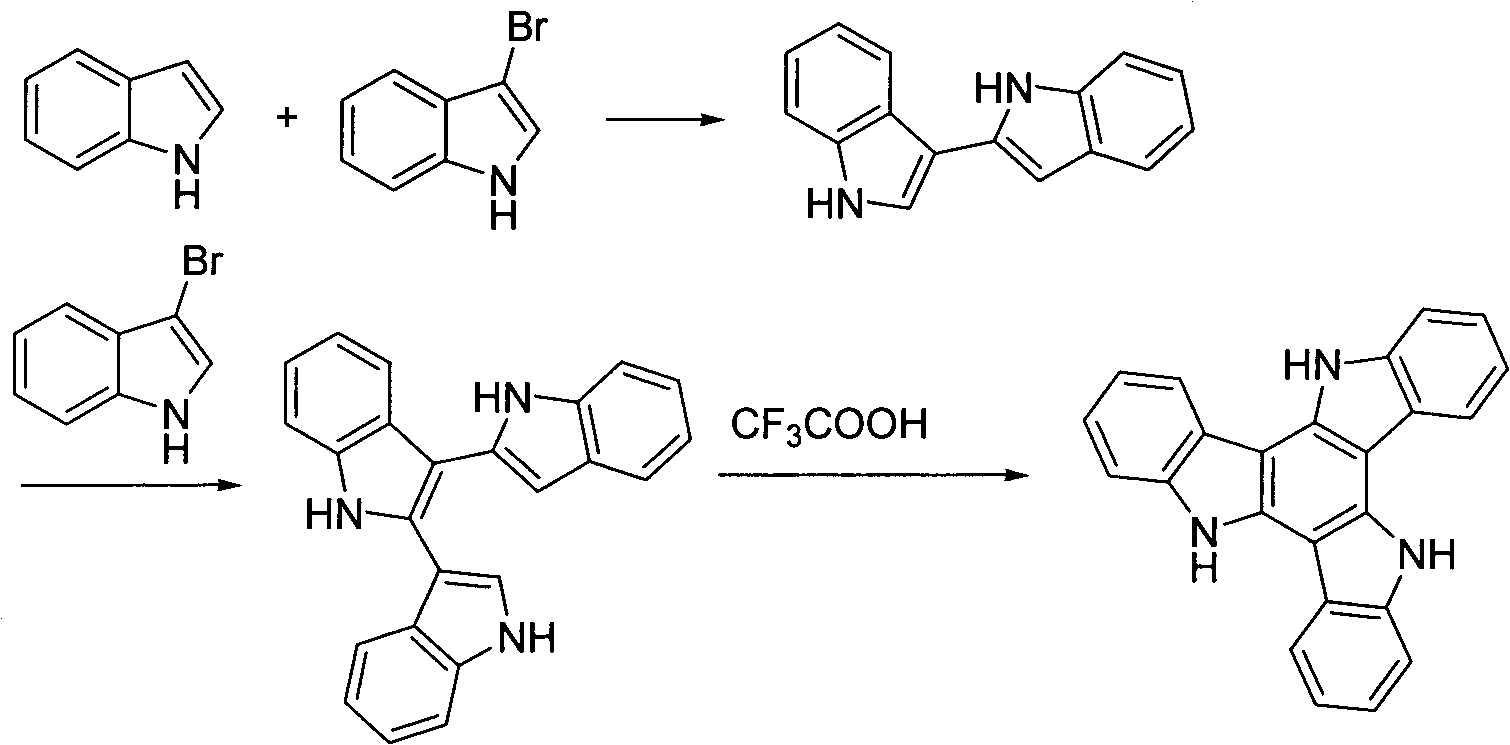
Method for preparing tricarbazole materials from aryl oxindole in one step
A technology of trioxacarbazoles and arylindolinones, which is applied in the direction of luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problem of long synthesis steps, single substituent mode and position, and restrictions on trioxacarbazoles Compound application and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Synthesis of 5,10,15-triethyl-3,8,13-triphenyltricarbazole (1a)
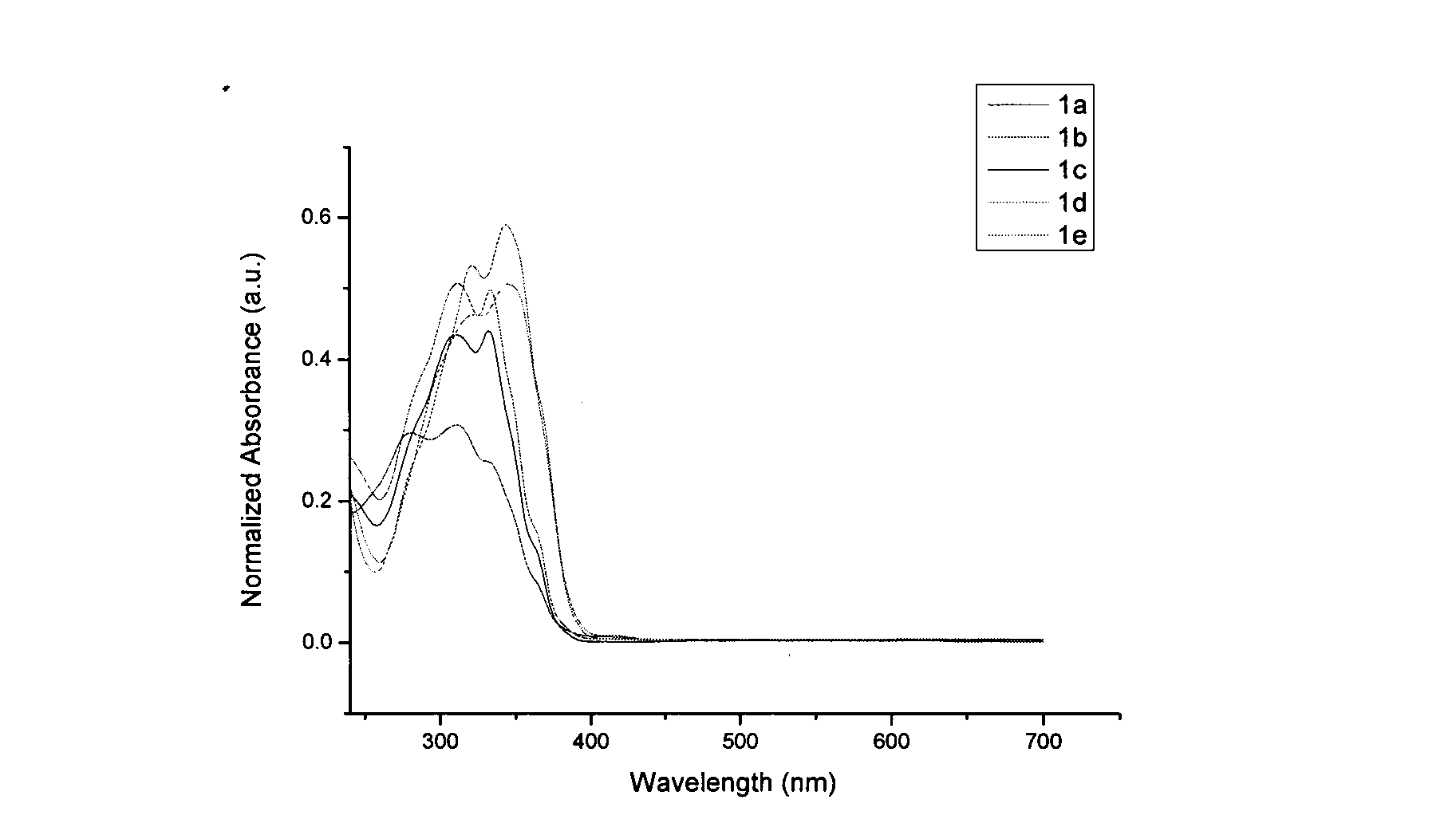
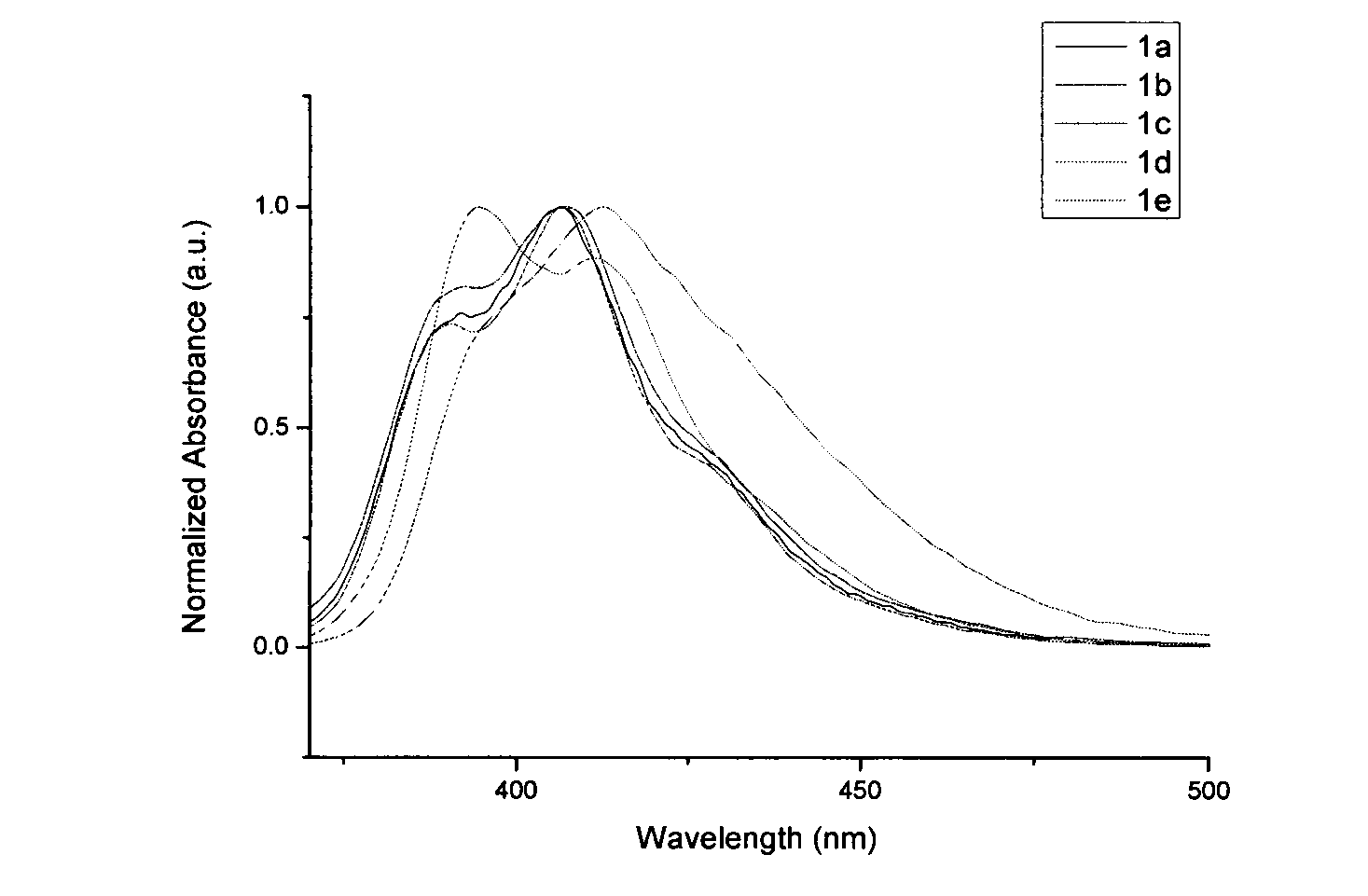
[0028] N-Ethyl-5-phenylindolinone (2.37g, 10.0mmol) was dissolved in 10ml of toluene, added to 10ml of phosphorus oxychloride solution, magnetically stirred, temperature controlled at 100-110°C, and reacted for 16h. Then cool to room temperature, pour into ice water, stir well, and use Na 2 CO 3 Neutralized to neutral, then extracted 3 times with dichloromethane, combined organic layers, dried, evaporated to remove solvent, and obtained white solid powder 5,10,15-triethyl-3,8,13-tri Phenyltricarbazole (1a) 0.85g, yield 39%, melting point 254.3°C, see attached figure 1 , the fluorescence emission spectrum is shown in the attached figure 2 .
Embodiment 2
[0029] Example 2: Synthesis of 5,10,15-triethyl-3,8,13-tri-tert-butylphenyltricarbazole (1b)
[0030] N-ethyl-5-tert-butylphenylindolinone (2.93g, 10.0mmol) was dissolved in 10ml of toluene, added to 10ml of phosphorus oxychloride solution, magnetically stirred, temperature controlled at 100-110°C, and reacted for 20h. Then cool to room temperature, pour into ice water, stir well, and use Na 2 CO 3 Neutralized to neutral, then extracted 3 times with dichloromethane, combined organic layers, dried, evaporated to remove solvent, and obtained white solid powder 5,10,15-triethyl-3,8,13-tri tert-butylphenyltricarbazole (1b) 1.11g, yield 40.5%, melting point 254.8°C, see attached figure 1 , the fluorescence emission spectrum is shown in the attached figure 2 .
Embodiment 3
[0031] Example 3: Synthesis of 5,10,15-triethyl-3,8,13-trimethoxyphenyltricarbazole (1c)
[0032] N-ethyl-5-methoxyphenylindolinone (2.67g, 10.0mmol) was dissolved in 10ml of toluene, added to 10ml of phosphorus oxychloride solution, magnetically stirred, temperature controlled at 100-110°C, and reacted for 10h. Then cool to room temperature, pour into ice water, stir well, and use Na 2 CO 3 Neutralized to neutral, then extracted 3 times with dichloromethane, combined organic layers, dried, evaporated to remove solvent, and obtained light yellow solid powder 5,10,15-triethyl-3,8,13- Trimethoxyphenyl tricarbazole (1c) 0.84g, yield 33.7%, melting point 367.8°C, see attached for ultraviolet absorption spectrum figure 1 , the fluorescence emission spectrum is shown in the attached figure 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com