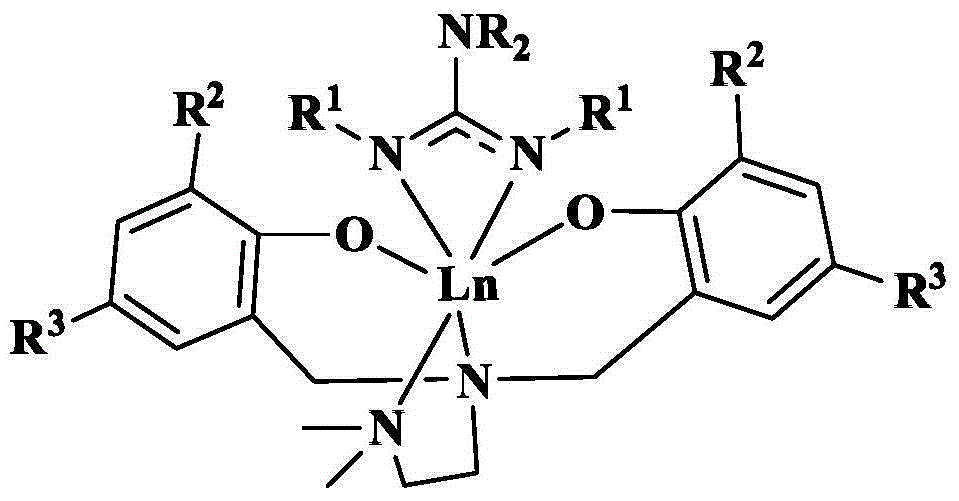
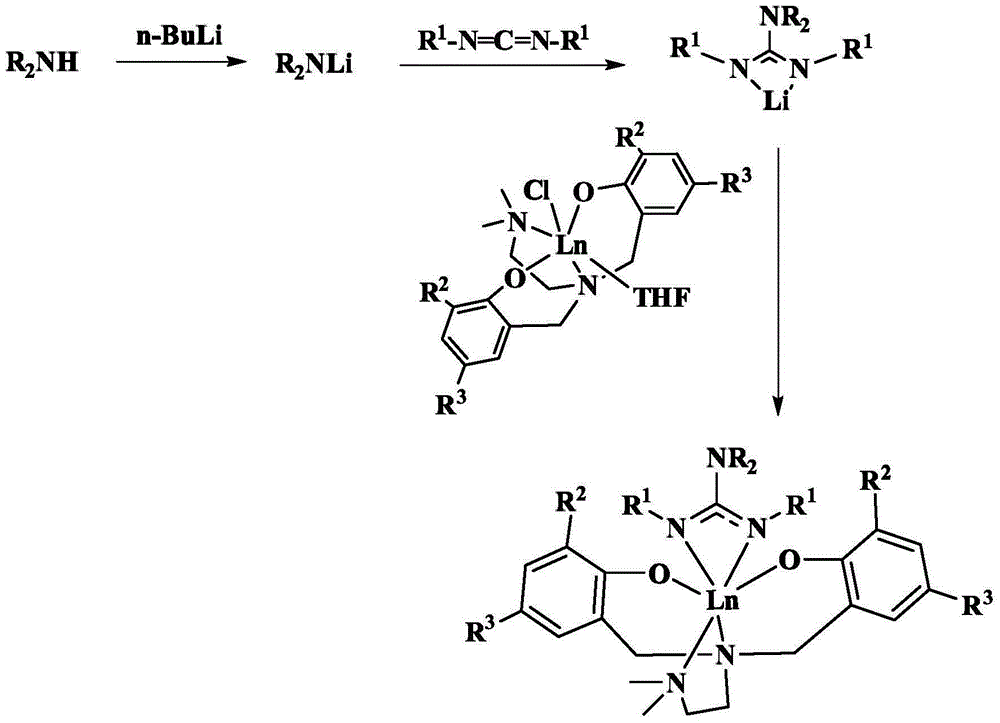
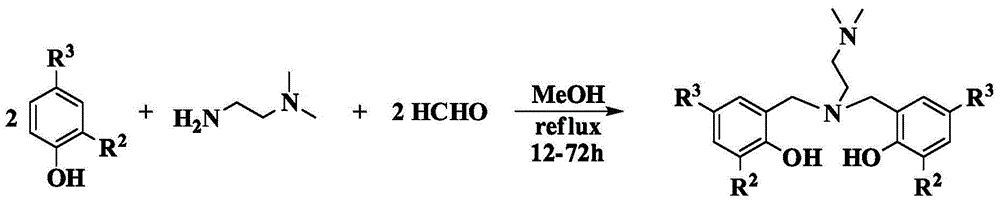
Amine bridged bisaryloxy rare earth metal guanidinium compound and its preparation method and application
A double aryloxy rare earth and rare earth metal technology is applied in the field of amine bridged double aryloxy rare earth metal guanidine compounds and their preparation, which can solve the problems of rare rare earth metal organic catalyst reports and a conversion rate of only 25%. , to achieve the effect of high molecular weight, fast reaction rate and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Preparation of [ONNO]Yb[Ph 2 NC(N i Pr) 2 ](R 2 =R 3 = Bu t )
[0056] Weigh 0.66 g (3.9 mmol) of diphenylamine into a reaction flask treated with dehydration and deoxygenation, add 20 ml of tetrahydrofuran (THF), place the bottle in an ice bath, add butyllithium n-BuLi (1.95 ml, 3.9 millimole, 2 mol / L hexane solution), after reacting for 2 hours; adding diisopropylcarbodiimide (0.49 g, 3.9 mmol), slowly rising to room temperature; after reacting for 1 hour, the above solution Add to [ONNO]YbCl(THF) (3.13 g, 3.9 mmol) in tetrahydrofuran (20 mL), and stir overnight at room temperature. After vacuuming the solvent, extract with toluene and centrifuge to remove lithium chloride (LiCl) precipitate; concentrate the toluene solution and let it stand at room temperature, two days later, yellow crystals (2.51 g, yield 65%) are precipitated. C 53 h 78 N 5 o 2 Theoretical value of Yb (990.26): C, 64.28; H, 7.94; N, 7.07. Elemental analysis: C, 64.69; H, 7.82...
Embodiment 2
[0057] Embodiment two: preparation [ONNO]Y[ i PR 2 NC(N i Pr) 2 ](R 2 =R 3 = Bu t )
[0058] Weigh 0.39 g (3.9 mmol) of diisopropylamine in the dehydration and deoxygenation treated reaction flask, add 20 ml of tetrahydrofuran, place the bottle in ice bath condition, add butyl lithium (1.95 ml, 3.9 mmol, 2 mole hexane solution per liter), after 2 hours of reaction, diisopropylcarbodiimide (0.49 g, 3.9 mmol) was added and slowly raised to room temperature; after 1 hour of reaction, the above solution was added to [ONNO] A solution of YCl(THF) (2.81 g, 3.9 mmol) in THF (20 mL) was stirred overnight at room temperature. After the solvent was dried in vacuum, it was extracted with toluene, and the precipitate was removed by centrifugation; the toluene solution was concentrated and allowed to stand at room temperature, and yellow crystals (1.63 g, yield 50%) were precipitated two days later. C 47 h 82 N 5 o 2 Theoretical value of Y (838.09): C, 67.36; H, 9.86; N, 8.36. ...
Embodiment 3
[0059] Embodiment three: preparation [ONNO]Y[ i PR 2 NC(NCy) 2 ](R 2 =R 3 = Bu t )
[0060] Weigh 0.38 g (3.8 mmol) of diisopropylamine in the reaction flask that has been dehydrated and deoxygenated, add 20 ml of tetrahydrofuran, place the bottle in an ice bath, add butyllithium (1.9 ml, 3.8 mmol, 2 mole hexane solution per liter), after 2 hours of reaction, dicyclohexylcarbodiimide (0.78 g, 3.8 mmol) was added, and slowly raised to room temperature; after 1 hour of reaction, the above solution was added to [ONNO]YCl (THF) (2.73 g, 3.8 mmol) in tetrahydrofuran (20 mL), stirred overnight at room temperature. After the solvent was dried in vacuum, it was extracted with toluene, and the precipitate was removed by centrifugation; the toluene solution was concentrated and allowed to stand at room temperature, and yellow crystals (1.92 g, yield 55%) were precipitated two days later. C 53 h 90 N 5 o 2 Theoretical value of Y (918.22): C, 69.33; H, 9.88; N, 7.63. Elemental...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com