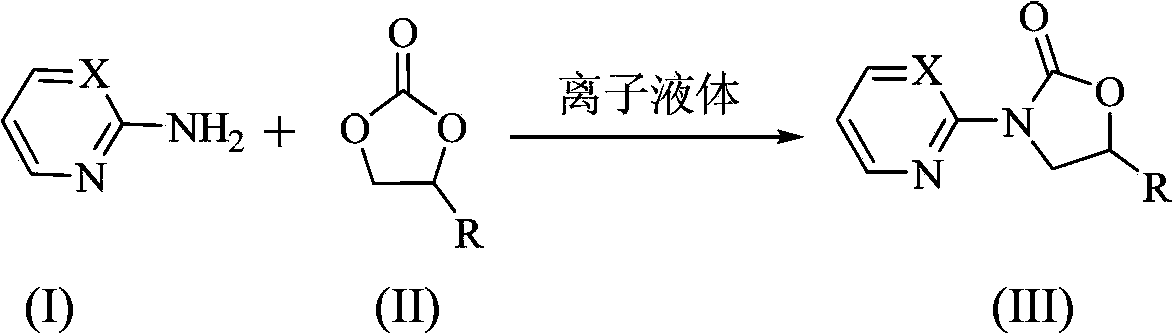
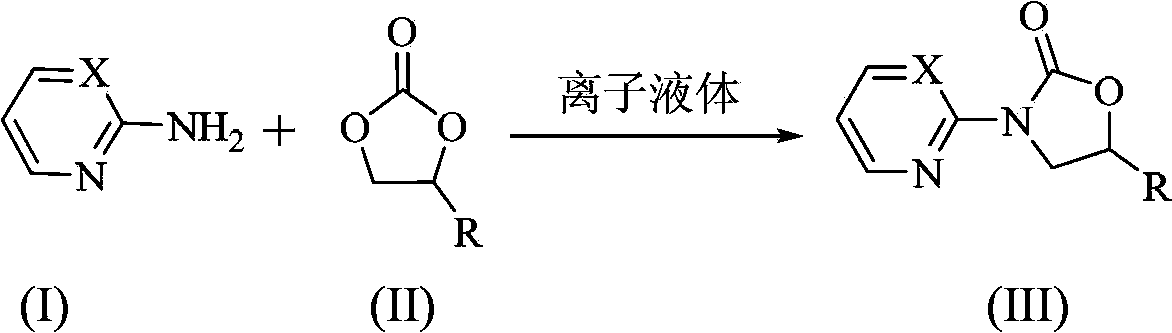
Preparation method of N-heterocyclic oxazolidine-2-ketone compound
A technology of heterocyclic oxazolidine and ketone compounds, which is applied in the field of preparation of N-heterocyclic oxazolidin-2-one compounds, can solve the problems of high cost, troublesome post-processing, complicated reaction process, etc., and achieve high The effect of universality, simple operation, high selectivity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] N 2 Under protection, 2-aminopyridine 2mmol (0.19g), ethylene carbonate 10mmol (0.88g), 1-butyl-3-methylimidazolium acetate ionic liquid ([Bmim]OAc ) 0.2mmol (0.04g), heated up to 130°C and stirred for 9h, cooled to room temperature after the reaction, the product was recrystallized from ethyl acetate, and dried in vacuo to obtain a white solid 3-(2-pyridyl)oxazolidine- 2-ketone 0.31g, yield 95%.
Embodiment 2
[0025] In the preparation method of this embodiment, the ionic liquid is 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF 4 ), the reaction time was 12h, other preparation conditions and methods were the same as in Example 1, and 0.17g of 3-(2-pyridyl)oxazolidin-2-one was prepared with a yield of 50%.
Embodiment 3
[0027] In the preparation method of this example, the ionic liquid is 1-butyl-3-methylimidazolium bromide ([Bmim]Br), other preparation conditions and methods are the same as in Example 1, and 3-(2-pyridyl)oxa Oxazolidin-2-one 0.29g, yield 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com