Copper difluorocarbene reagent, and preparation method and application thereof
A difluorocarbene and copper reagent technology, applied in the difluorocarbene copper reagent and its preparation and application fields, can solve the problems of limited wide use, general reactivity, inconvenient operation, etc., and achieve low production cost, simple and convenient reaction operation, good The effect of industrial application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
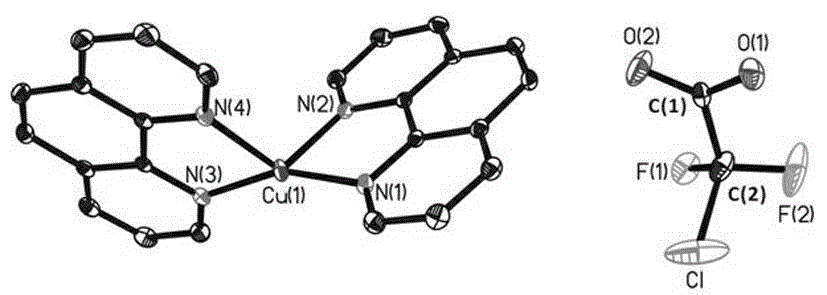
[0020] Under the protection of nitrogen, add a polytetrafluoroethylene magnetic stirring bar to the reactor, add 10.00 mmol cuprous chloride to it, add 10 mL tetrahydrofuran solvent and mix well, then add dropwise 55 mL containing 12.00 mmol tert-butyl The tetrahydrofuran solution of sodium alkoxide was stirred at room temperature for 25 minutes, filtered to obtain a light yellow clear solution, and 15 mL of tetrahydrofuran solution containing 14.00 mmol o-phenanthroline was added dropwise to obtain a dark reddish-brown solution. Slowly add 10 mL of tetrahydrofuran solution containing 7.00 mmol of difluorochloroacetic acid dropwise into the solution. After the dropwise addition, continue stirring at room temperature for 5 minutes; filter out the resulting precipitate, and wash the resulting precipitate with ether to obtain a dark reddish-brown solid After recrystallization, reddish-brown granular crystals were obtained, namely 1,10-phenanthroline-difluorochloroacetic acid cupro...
Embodiment 2
[0022] Under the protection of nitrogen, add a polytetrafluoroethylene magnetic stirring bar to the reactor, add 10.00 mmol cuprous chloride to it, add 10 mL tetrahydrofuran solvent and mix well, then add dropwise 55 mL containing 12.00 mmol tert-butyl The tetrahydrofuran solution of sodium alkoxide was stirred at room temperature for 25 minutes, filtered to obtain a light yellow clear solution, and 15 mL of tetrahydrofuran solution containing 14.00 mmol 2,2-bipyridine was added dropwise to obtain a dark reddish-brown solution. Slowly add 10 mL of tetrahydrofuran solution containing 7.00 mmol difluorochloroacetic acid dropwise into the reddish-brown solution. After the dropwise addition, continue to stir for 5 minutes at room temperature; Brown solid, reddish-brown granular crystals obtained after recrystallization, that is, 2,2-bipyridine difluorochloroacetic acid cuprous(I) complex (bpy)Cu(O 2 CCF 2 Cl), productive rate 75%, 1 H-NMR (400 MHz, DMSO- d 6 ) δ: 8.66 (d, J ...
Embodiment 3
[0024]Under the protection of nitrogen, add a polytetrafluoroethylene magnetic stirring bar to the reactor, add 10.00 mmol cuprous chloride to it, add 10 mL tetrahydrofuran solvent and mix well, then add dropwise 55 mL containing 12.00 mmol tert-butyl Sodium alkoxide solution in tetrahydrofuran, stirred at room temperature for 25 minutes, filtered to obtain a light yellow clear solution, dropwise added 15 mL of tetrahydrofuran solution containing 14.00 mmol 2,9-dimethyl-phenanthroline to obtain a dark reddish-brown solution, used once Slowly add 10 mL of tetrahydrofuran solution containing 7.00 mmol of difluorochloroacetic acid into the above dark reddish-brown solution with a syringe, and after the addition, continue to stir for 5 minutes at room temperature; After washing, a dark reddish brown solid was obtained, and after recrystallization, a reddish brown granular crystal was obtained, which was 2,9-dimethyl-phenanthroline-difluorochloroacetic acid cuprous (I) complex (2,9-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com