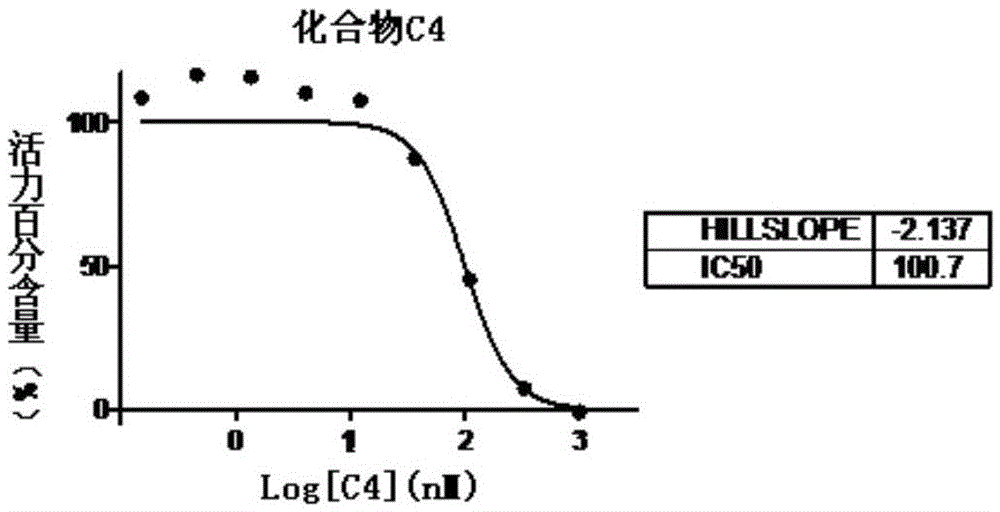
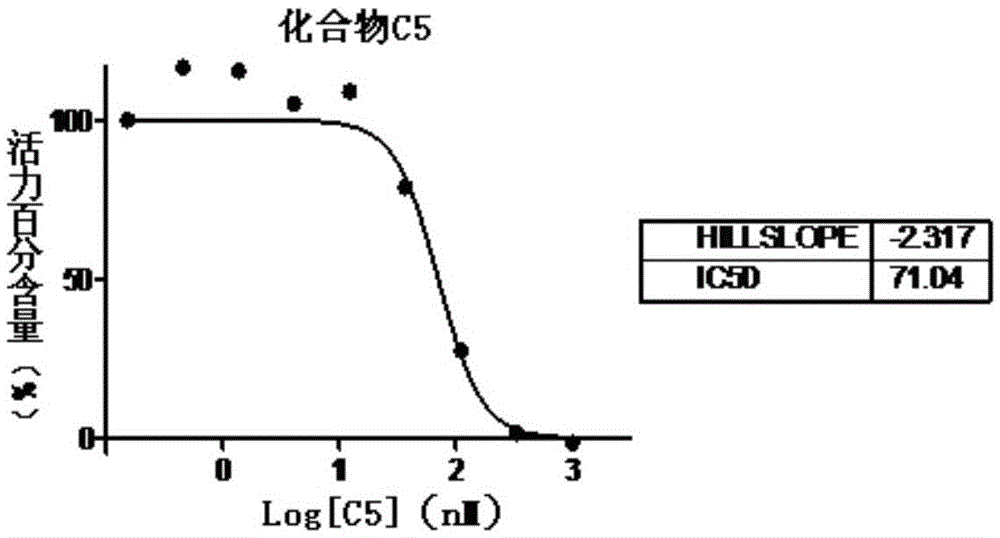
Aminothiazole-pyridine heterocyclic compounds with hedgehog pathway antagonist activity
A technology of aminothiazole and hedgehog pathway, applied in the field of pharmaceutical synthesis, can solve the problems of poor physical and chemical properties and low solubility of vismodegib, achieve the effect of blocking tumor cell metastasis and regeneration, and treating tumor diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] This embodiment provides an aminothiazole-pyridine heterocyclic compound C1, which is synthesized by the following method:
[0048]
[0049] 1) Synthesis of intermediate C1-1:
[0050] Add N-tert-butoxycarbonylpiperidone (20g, 100.5mmol), cyanamide (8.7g, 200.9mmol), sublimed sulfur (6.4g, 200mmol) into 100mL of pyridine, and react at 130°C for 90 minutes. After cooling to room temperature, crystals were precipitated, filtered, and the obtained solid was washed twice with diethyl ether (100 mL) to obtain a light yellow solid (19 g, 74.2%). 1 HNMR (400MHz, DMSO-d6) δ6.83 (s, 2H), 4.29 (s, 2H), 3.56 (s, 2H), 2.43 (s, 2H), 1.41 (s, 9H).
[0051] 2) Synthesis of intermediate C1-2:
[0052] Add C1-1 (200mg, 0.78mmol) to 3M hydrogen chloride in ethyl acetate (3mL), stir at room temperature for 3 hours, spin off the solvent under reduced pressure, and add the concentrate to saturated aqueous sodium bicarbonate (5mL) and dichloromethane (20mL), the organic phase was dried...
Embodiment 2
[0056] This embodiment provides an aminothiazole-pyridine heterocyclic compound C2, which is synthesized by the following method:
[0057]
[0058] The intermediate C1 was synthesized according to the method in Example 1, and then the product C2 was prepared.
[0059] C1 (40mg, 0.11mmol), acetic acid (9mg, 0.14mmol), N,N`-diisopropylethylamine (28mg, 0.22mmol) were dissolved in N,N`-dimethylformamide (1.5mL). HATU (53 mg, 0.14 mmol) was added to the solution and stirred at room temperature for 24 hours. The reaction solution was added into ethyl acetate (40 mL), and washed with saturated brine (15 mL×3). The organic phase was dried over anhydrous sodium sulfate and filtered, the filtrate was concentrated under reduced pressure, and purified by silica gel column (dichloromethane:methanol=70:1) to obtain yellow solid C2 (20 mg, 45%). 1 HNMR (400MHz, CDCl 3 )δ9.55(s,1H),8.35(s,1H),8.22(s,1H),7.43(s,1H),6.66(s,1H),4.68(s,2H),3.96(t,J =5.6Hz,2H),2.83(t,J=5.4Hz,2H),2.38(s,3H)...
Embodiment 3
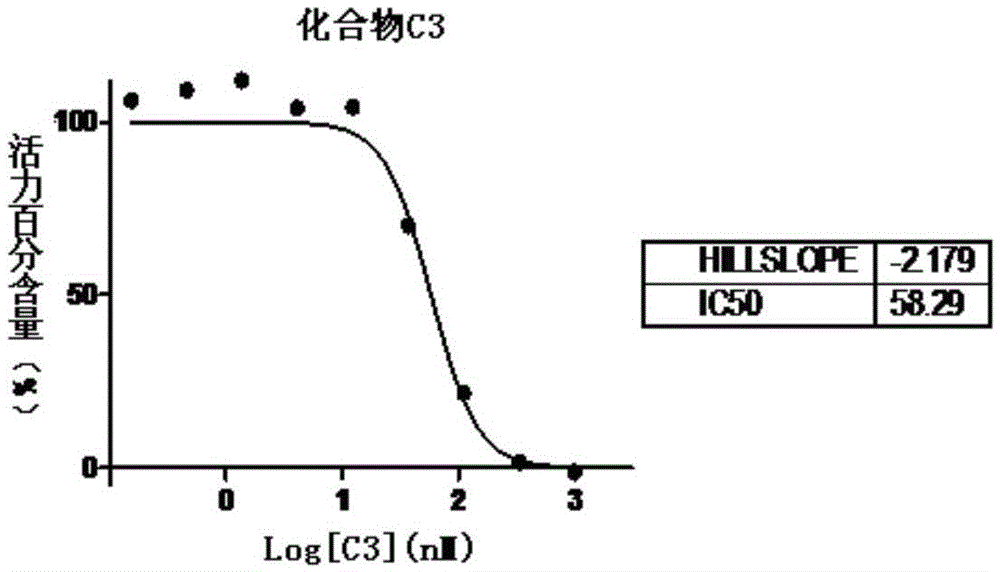
[0062] This embodiment provides an aminothiazole-pyridine heterocyclic compound C3, which is synthesized by the following method:
[0063]
[0064] The intermediate C1 was synthesized according to the method in Example 1, and then the product C3 was prepared.
[0065] C1 (35mg, 0.094mmol), isobutyric acid (11mg, 0.125mmol), N,N`-diisopropylethylamine (20mg, 0.155mmol) dissolved in N,N`-dimethylformamide (1.5mL ). HATU (49 mg, 0.129 mmol) was added to the solution and stirred at room temperature for 24 hours. The reaction solution was added into ethyl acetate (40 mL), and washed with saturated brine (15 mL×3). The organic phase was dried over anhydrous sodium sulfate and filtered, the filtrate was concentrated under reduced pressure, and purified by silica gel column (dichloromethane:methanol=80:1 to 50:1) to obtain white solid C3 (13.3 mg, 32%). 1 HNMR (400MHz, CDCl 3 )δ9.89(s,1H),8.36(s,1H),8.22(s,1H),7.43(s,1H),6.67(s,1H),4.67(s,2H),3.96(t,J =5.6Hz,2H),2.82(t,J=5.4Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com