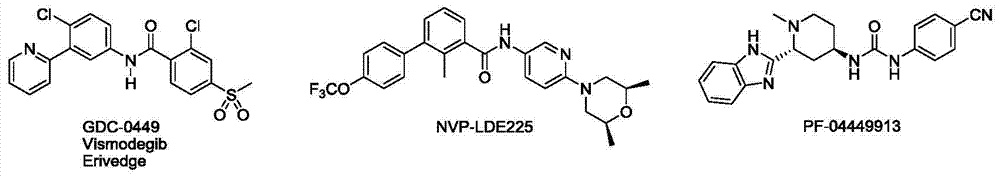
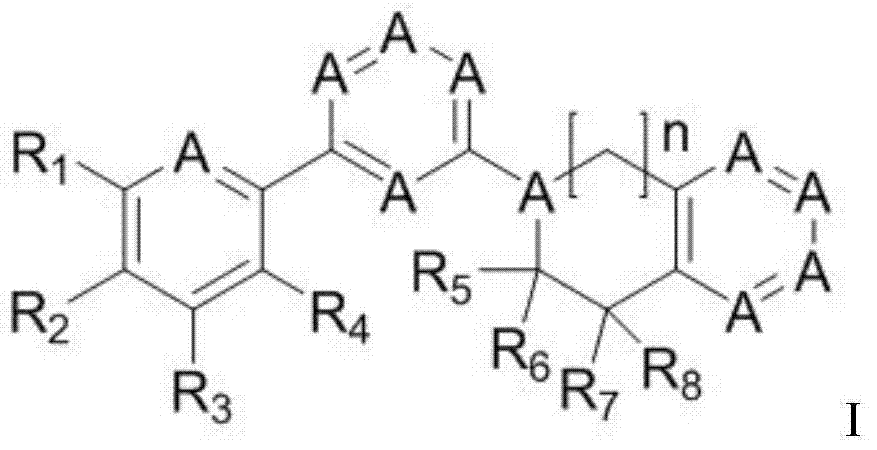
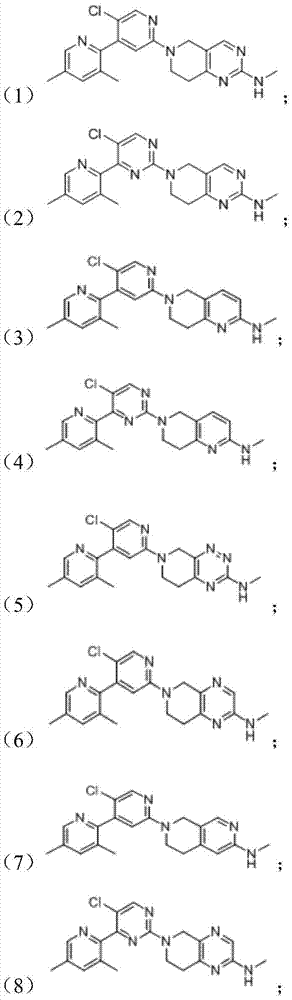
Pyrimidine antitumor compound with Hedgehog antagonist activity
A hedgehog pathway and compound technology, applied in the field of pyrimidine anti-tumor compounds, can solve the problems of tumor metastasis and regeneration with little efficacy, and achieve the effect of blocking metastasis and regeneration and inhibiting abnormal cell growth.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] This embodiment provides an antitumor compound A1, the synthesis method of which is as follows:
[0054]
[0055] 1) Synthesis of intermediate A1-2:
[0056] Dissolve 4-tert-butoxycarbonylpiperidone (A1-1, 10g, 50.2mmol) in 40ml of N,N-dimethylformamide, and add N,N-dimethylformamide dimethyl After the addition of acetal (6 g, 50 mmol), the reaction was carried out at 80°C for 12 hours. Cooled to room temperature, added to ethyl acetate (150mL) and water (50mL), the organic phase was washed twice with saturated brine (50mL), dried and filtered over anhydrous sodium sulfate, and rotary evaporated to obtain an orange crude product (13g). Vote for the next response.
[0057] 2) Synthesis of intermediate A1-3:
[0058] At room temperature, dissolve hemimethylthiourea sulfate (6.98g, 25.1mmol) and sodium ethoxide (3.28g, 40mmol) in 40ml of ethanol, stir for half an hour, then add the intermediate A1-2 (13g, 50.2 mmol) in 10 ml of ethanol solution, reflux for 12 h, coo...
Embodiment 2
[0071] This embodiment provides an antitumor compound A2, the synthesis method of which is as follows:
[0072]
[0073] 1) Synthesis of intermediate A2-1:
[0074] A1-4 (1g, 3.19mmol) and ethylamine aqueous solution (71%, 1mL, 15.8mmol) were successively dissolved in ethanol (10mL) under stirring, heated to reflux, after 12 hours, cooled to room temperature, and decompressed to spin off The solvent and the concentrate were purified by column chromatography (mobile phase: petroleum ether: ethyl acetate = 4:1) to obtain a white solid (400mg, 45%).
[0075] 2) Synthesis of intermediate A2-2:
[0076] Dissolve A2-1 (400mg, 1.44mmol) in a small amount of dichloromethane, add hydrogen chloride in saturated ethyl acetate solution (3mL), stir at room temperature for 3 hours, spin off the solvent under reduced pressure, and add the concentrate to saturated aqueous sodium bicarbonate solution (5mL) and dichloromethane (20mL), the organic phase was washed with saturated brine (10mL...
Embodiment 3
[0081] This embodiment provides an antitumor compound A3, the synthesis method of which is as follows:
[0082]
[0083] 1) Synthesis of intermediate A3-1:
[0084] A1-4 (1g, 3.19mmol) and cyclopropylamine (300mg, 5.26mmol) were successively dissolved in ethanol (10mL) under stirring, heated to reflux, after 12 hours, cooled to room temperature, decompressed to spin off the solvent, and concentrate After purification by column chromatography (mobile phase: petroleum ether: ethyl acetate = 4:1), a white solid (460mg, 50%) was obtained.
[0085] 2) Synthesis of intermediate A3-2:
[0086] Dissolve A3-1 (460mg, 1.58mmol) in a small amount of dichloromethane, add hydrogen chloride in saturated ethyl acetate solution (3mL), stir at room temperature for 3 hours, spin off the solvent under reduced pressure, and add the concentrate to saturated aqueous sodium bicarbonate solution (5 mL) and dichloromethane (20 mL), the organic phase was washed with saturated brine (10 mL), dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com