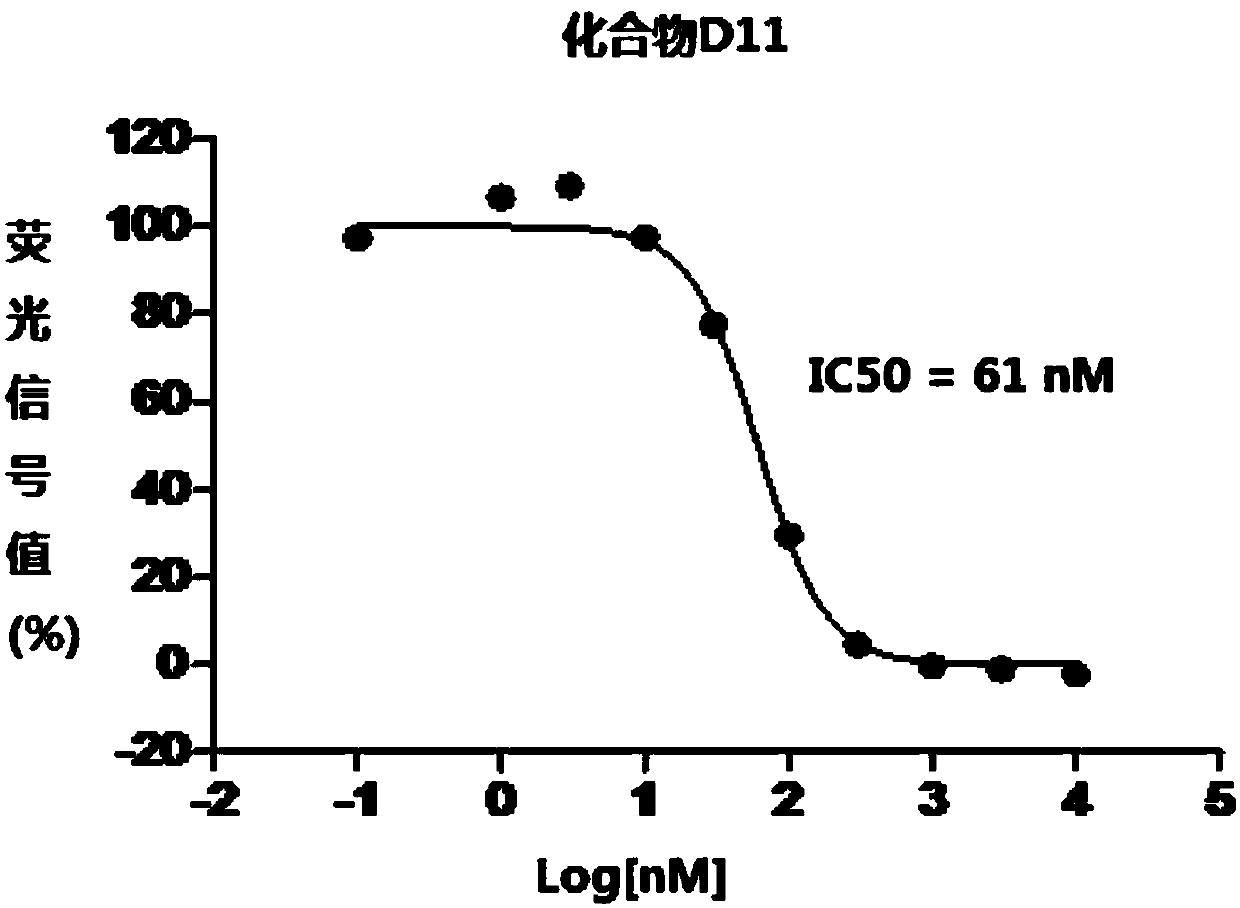
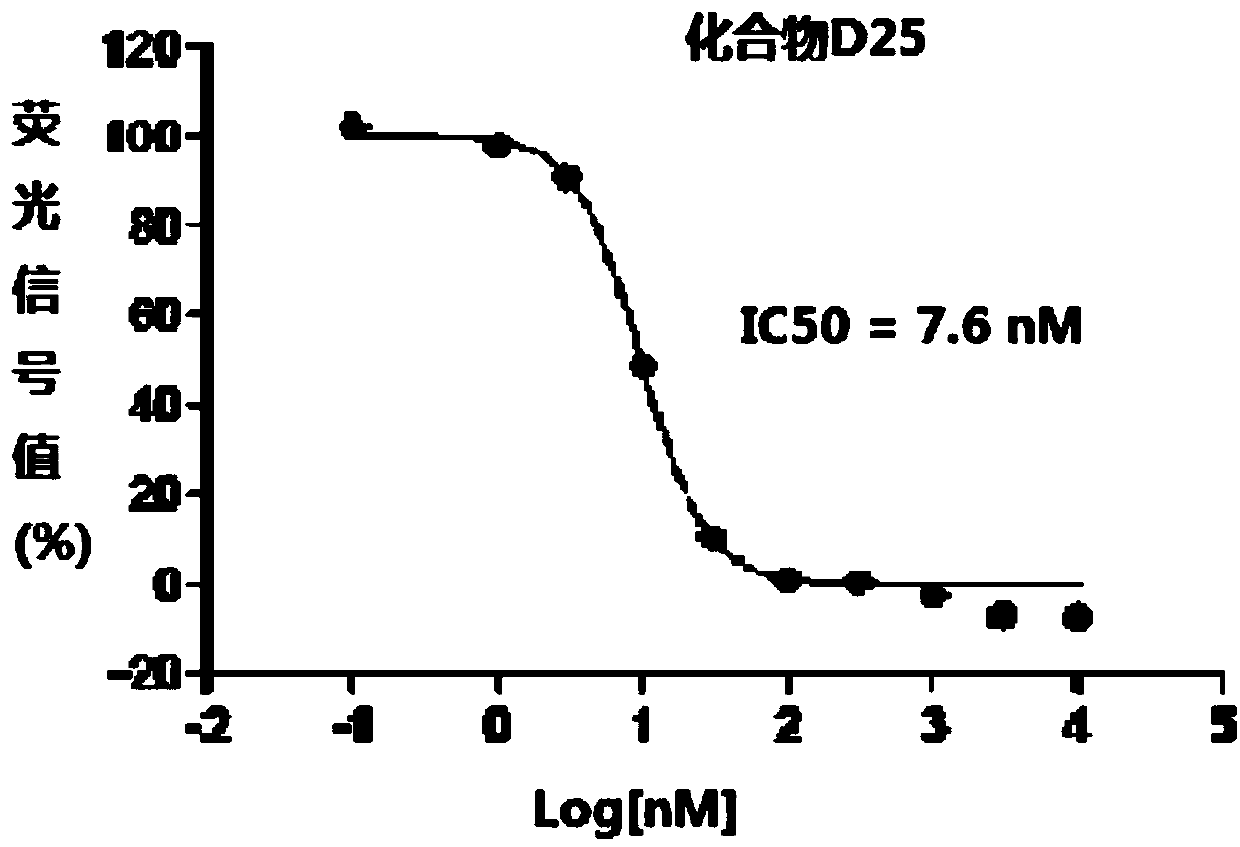
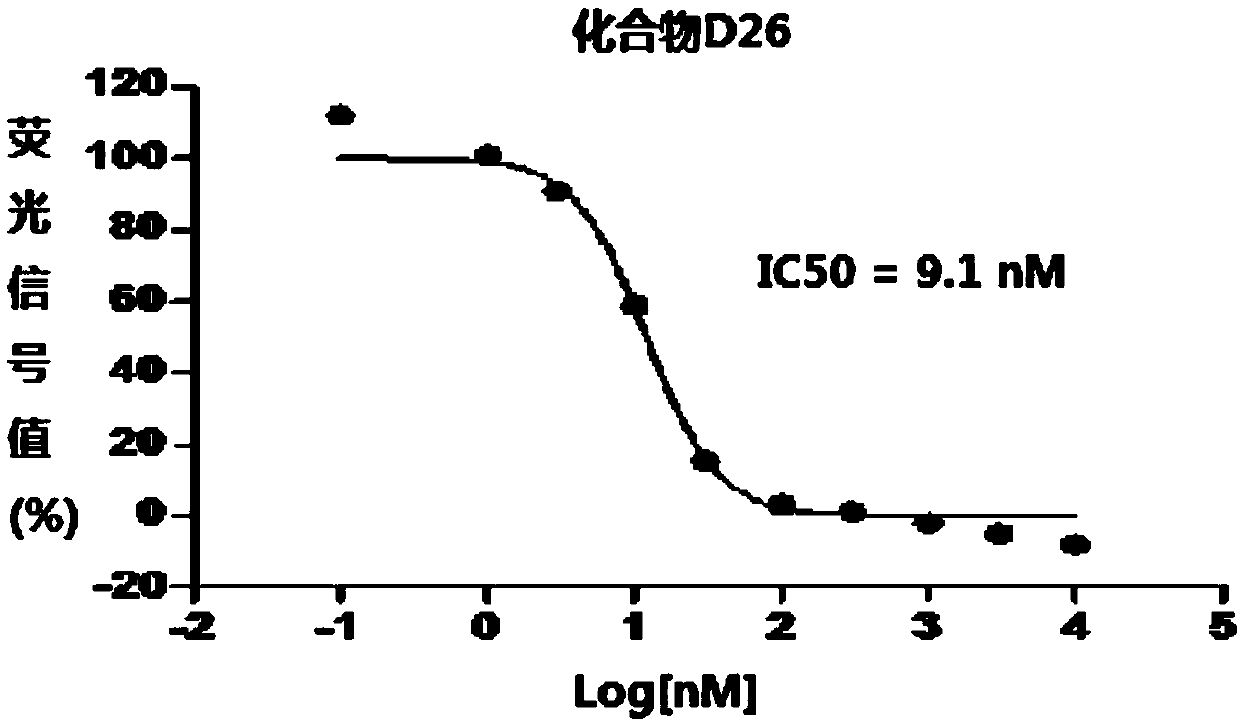
1,3,4-thiadiazole heterocyclic compound having hedgehog pathway antagonist activity
A technology of heterocyclic compounds and hedgehog pathway, applied in the field of 1,3,4-thiadiazole heterocyclic compounds, can solve the problems of low solubility and poor physical and chemical properties of vismodegib, and achieve the treatment of tumor diseases and block the metastasis and regeneration of tumor cells. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This example provides a 1,3,4-thiadiazole heterocyclic compound D1 with hedgehog signaling pathway antagonistic activity, which is synthesized by the following method:
[0044]
[0045] 1) Synthesis of intermediate D1-1:
[0046] 2-Amino 1,3,4-thiadiazole (10g, 100mmol) and sodium bicarbonate (16g, 190mmol) were added to methanol (140mL), and bromine (5.5mL, 110mmol) was slowly added dropwise at 0°C ), and then stirred at this temperature for 0.5 hours. Concentrate under reduced pressure to remove most of the methanol, add water (150 mL) to the reaction system to dilute, filter, and vacuum dry to obtain a brown solid (16 g, 89%). 1 H NMR (400MHz, DMSO-d6) δ7.51 (s, 2H).
[0047] 2) Synthesis of intermediate D1-2:
[0048] Intermediate D1-1 (5.0g, 27.8mmol), ethyl 4-piperidinecarboxylate (5.2g, 33.3mmol) and potassium carbonate (7.7g, 55.6mmol) were added to isopropanol (100mL) at 90 Stir at °C for 3 hours. Cool to room temperature, concentrate under reduced pres...
Embodiment 2
[0060] This example provides a 1,3,4-thiadiazole heterocyclic compound D2 with hedgehog signaling pathway antagonistic activity, which is synthesized by the following method:
[0061]
[0062] Intermediate D1-6 (80mg, 0.2mmol), isopropylamine (18mg, 0.3mmol), DIPEA (77mg, 0.6mmol) and HATU (91mg, 0.24mmol) were added to DMF (1mL), stirred at room temperature for 12 hours . The reaction solution was diluted with ethyl acetate (50 mL), and washed with saturated aqueous sodium chloride solution (30 mL*5). The organic phase was dried over anhydrous sodium sulfate, filtered, concentrated, and subjected to column chromatography (petroleum ether: ethyl acetate = 2:1) to obtain a white solid (25 mg, 28%). After nuclear magnetic spectrum analysis (see Table 1 for spectral data), the obtained solid was compound D2.
Embodiment 3
[0064] This example provides a 1,3,4-thiadiazole heterocyclic compound D3 with hedgehog signaling pathway antagonistic activity, which is synthesized by the following method:
[0065]
[0066] Intermediate D1-6 (70mg, 0.18mmol), cyclopropylamine (15mg, 0.26mmol), DIPEA (70mg, 0.54mmol) and HATU (82mg, 0.22mmol) were added to DMF (1mL), stirred at room temperature for 12 hours . The reaction solution was diluted with ethyl acetate (50 mL), and washed with saturated aqueous sodium chloride solution (30 mL*5). The organic phase was dried over anhydrous sodium sulfate, filtered, concentrated, and subjected to column chromatography (petroleum ether: ethyl acetate = 2:1) to obtain a yellow solid (45 mg, 58%). After nuclear magnetic spectrum analysis (see Table 1 for spectral data), the obtained solid was compound D3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com