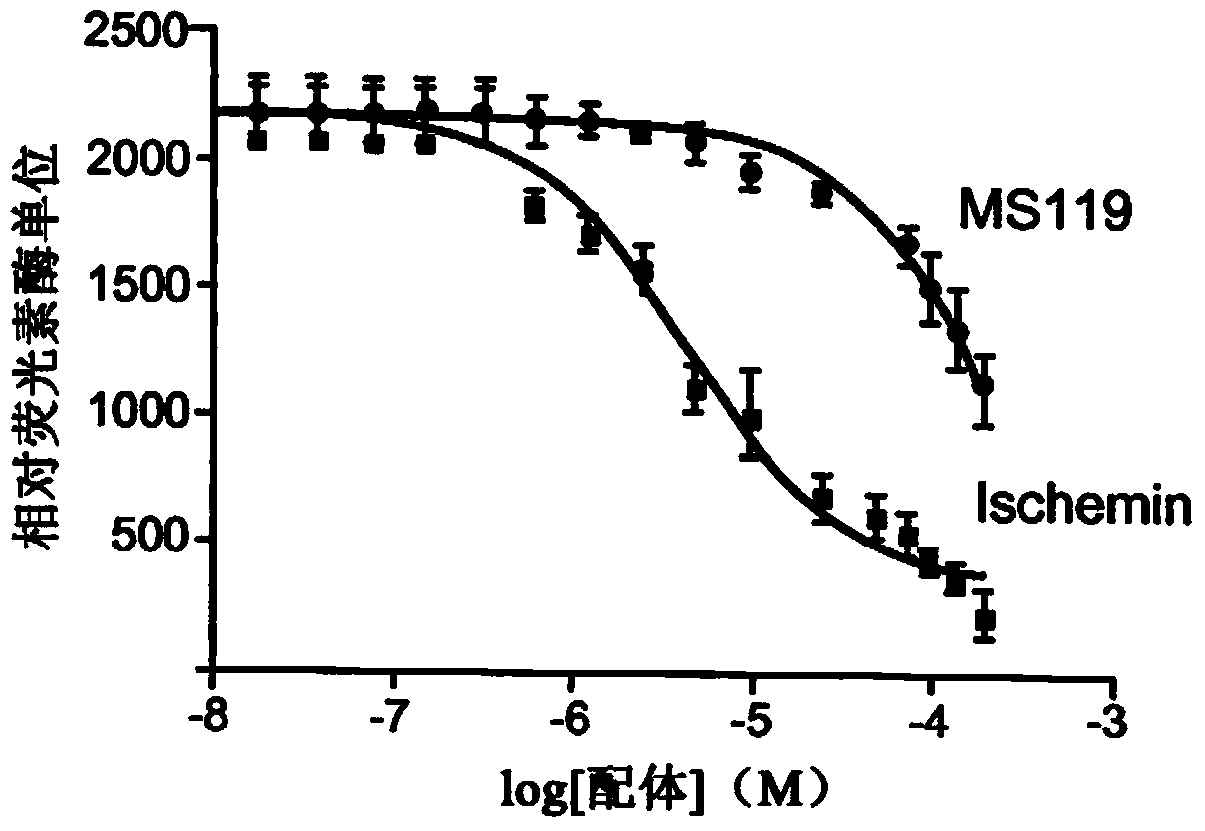
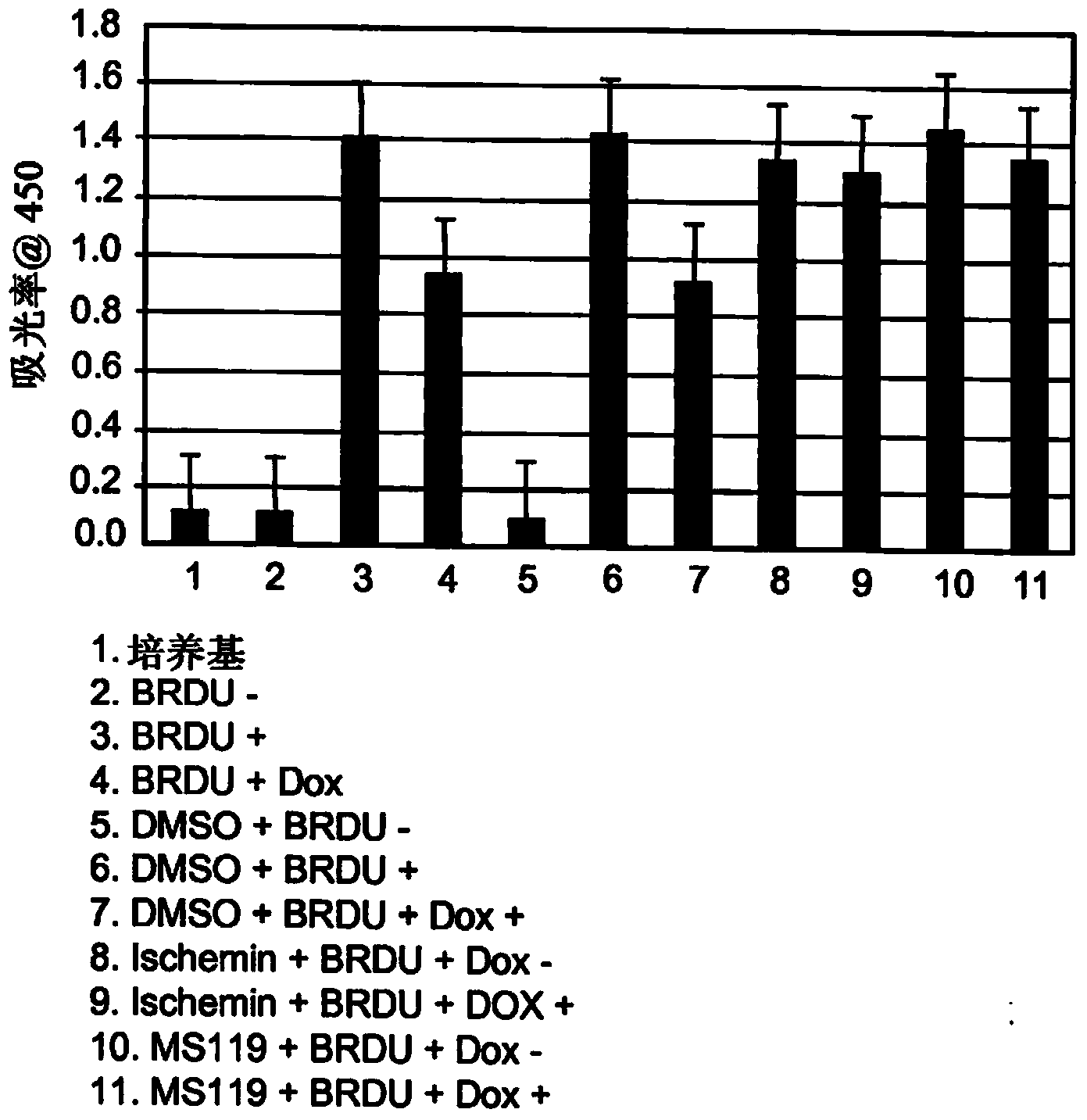
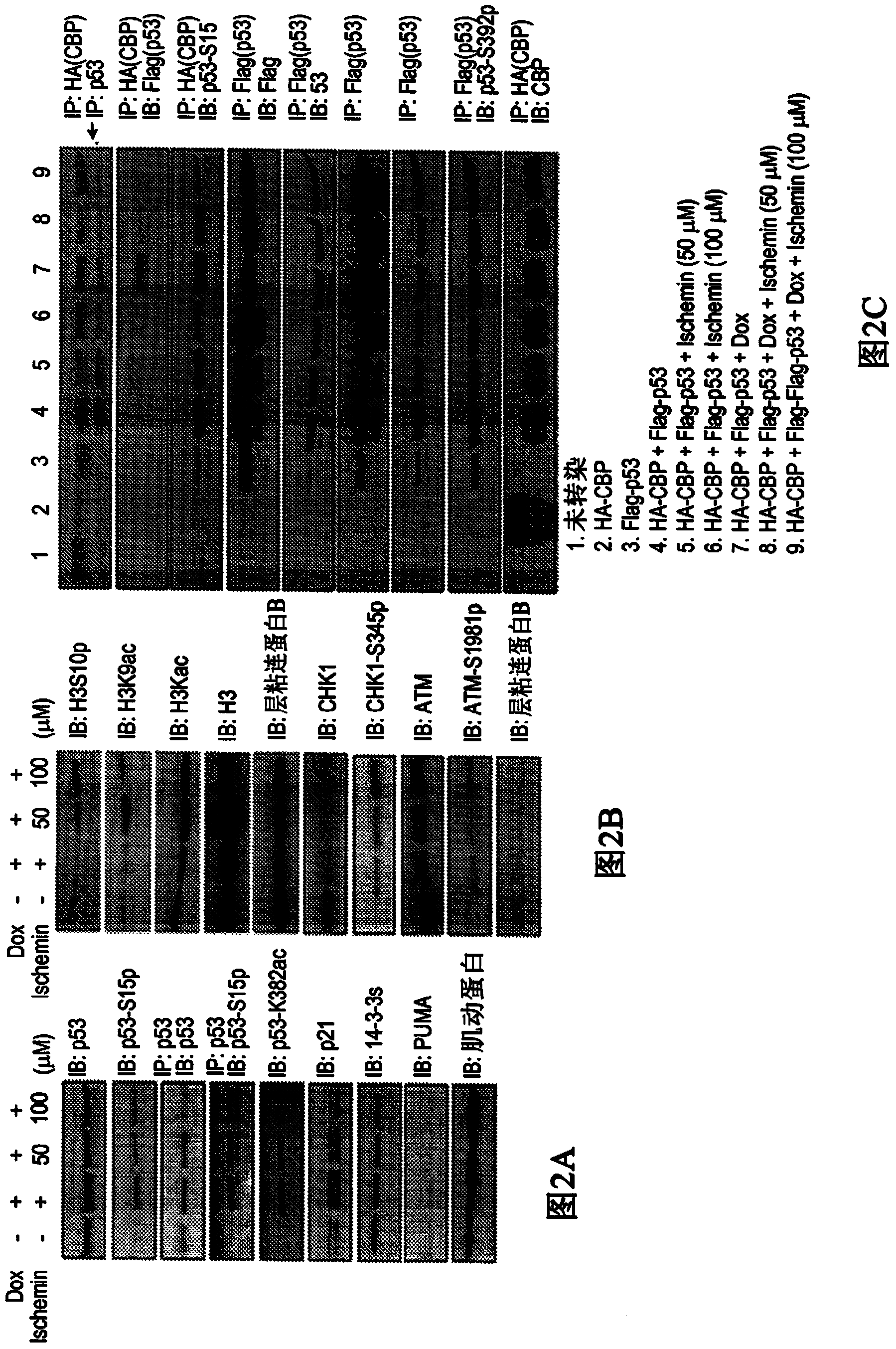
Inhibitors of bromodomains as modulators of gene expression
A group and alkyl technology, applied in the field of inhibitors of bromodomain proteins as regulators of gene expression, can solve problems such as unfavorable hyperactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0158] Preparation of compounds may involve protection and deprotection of various chemical groups. The need for protection and deprotection and the choice of suitable protecting groups can be readily selected by one skilled in the art. The chemistry of protecting groups can be found, for example, in: Protecting Group Chemistry, First Edition, Oxford University Press, 2000; and March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, Fifth Edition, Wiley-Interscience Publication, 2001 (each of which is incorporated herein by reference in its entirety).
[0159] The reaction can be monitored by any suitable method in the art, for example product formation can be monitored by spectroscopic methods, such as nuclear magnetic resonance spectroscopy (e.g. 1 H or 13 C), infrared spectroscopy, spectrophotometry (UV-visible), mass spectrometry; or by chromatography, such as high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LCMS) ...
Embodiment 1
[0290] Embodiment 1. Preparation of the compound represented by formula (1).
[0291] A. Steps for preparing building blocks and intermediates of compounds represented by formula (1).
[0292]
[0293] Reference: BMCL 2008, 18(23)6093-6096
[0294] A solution of 2-aminopyridine (1.0 g, 10.6 mmol) in pyridine (5 mL) was cooled to 0°C and treated in portions with p-iodobenzenesulfonyl chloride (3.37 g, 11.2 mmol). The solution was heated to 60°C for 1 hour and then cooled to 25°C. Most of the solvent was removed under vacuum, and the residue was dissolved in a small amount of MeOH (20 mL) and H 2 O (100mL) suspended. The white solid that had formed was collected by suction filtration. The solid is dissolved in a small amount of CH 2 Cl 2 , and precipitated by adding hexane to give the final compound as a white solid (3.34 g, 87%) which was used without further purification. 1 H NMR (600MHz, DMSO-d 6 )δ7.97(1H,d,J=4.8Hz),7.91(2H,d,J=8.4Hz),7.75(1H,t,J=7.2Hz),7.61(2H,d,...
Embodiment CM278
[0303]
[0304] Will be in DMF:Et 3 A solution of starting material (0.807 g, 2.24 mmol, 1.1 equiv, iodide) in N (6.0 mL) was treated with Pd(OAc) 2 (0.091g, 0.406mmol, 0.02 equivalent), P-(o-tolyl) 3 (0.371g, 1.22mmol, 0.06eq) and the alkene product (0.508g, 2.03mmol, 1eq) worked up. The solution was heated to 100° C. for 2 hours in a microwave tube. The resulting solution was then filtered, concentrated in vacuo and purified by flash chromatography (0-3% MeOH-CH 2 Cl 2 ) to give CM278 (1.11 g, 99%) as a clear oil. 1 H NMR (600MHz, CDCl 3 )δ8.36(1H,d,J=5.4Hz),7.89(2H,d,J=8.4Hz),7.71(1H,t,J=7.8Hz),7.53(2H,d,J=8.4Hz) ,7.45(1H,d,J=9.0Hz),7.30(1H,s),7.16(1H,d,J=16.2Hz),6.86(1H,d,J=16.2Hz),6.83(1H,t, J=6.0Hz),6.52(1H,s),2.18(3H,s),1.51(9H,s).LCMS m / z482.1496([M+H + ],C 25 h 27 N 3 o 5 S requires 482.1744).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com