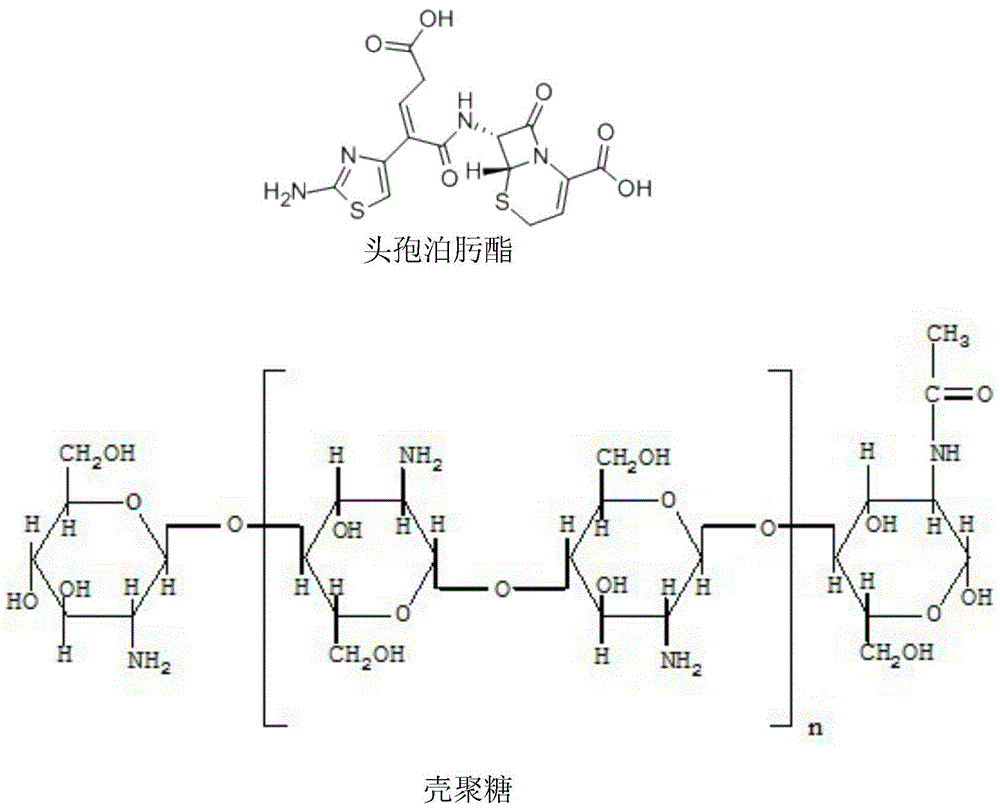
Cefpodoxime proxetil composition freeze-dried powder injection for injection
A technology of cefpodoxime axetil and freeze-dried powder injection, which is applied in the field of medicine and medicine manufacturing, can solve the problems of not containing chitosan nanoparticles, avoid nephrotoxicity and hemolysis, reduce dosage, and be beneficial to clinical application Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1. Preparation of lyophilized powder injection of cefpodoxime axetil composition for injection (specification: 100 mg, calculated as cefpodoxime axetil), in 1000 vials.
[0035] 1. Prescription:
[0036] Cefpodoxime Proxetil 100g
[0037] Chitosan Nanoparticles 50g
[0038] Water for injection 2000ml
[0039] 2. Preparation process:
[0040] 1) Slowly add the prescribed amount of chitosan nanoparticles into the prescribed amount of water for injection, and stir until dissolved while adding.
[0041] 2) Add the prescribed amount of cefpodoxime axetil and stir to dissolve until clear.
[0042] 3) Use sodium hydroxide to adjust the pH to 6.0, add 0.1% activated carbon and stir for 30 minutes, filter out the activated carbon, and filter the liquid through 0.45 μm and 0.22 μm microporous membranes to detect the content of intermediates, calculated as cefpodoxime axetil 0.2g per bottle is calculated as filling volume.
[0043] 4) Fill according to the test re...
Embodiment 2
[0044] Example 2. Preparation of lyophilized powder injection of cefpodoxime axetil composition for injection (specification: 100 mg, calculated as cefpodoxime axetil), calculated in 1000 vials.
[0045] 1. Prescription:
[0046] Cefpodoxime Proxetil 100g
[0047] Chitosan Nanoparticles 60g
[0048] Water for injection 2000ml
[0049] 2. Preparation process:
[0050] 1) Slowly add the prescribed amount of chitosan nanoparticles into the prescribed amount of water for injection, and stir until dissolved while adding.
[0051] 2) Add the prescribed amount of cefpodoxime axetil and stir to dissolve until clear.
[0052] 3) Use sodium hydroxide to adjust the pH to 6.0, add 0.1% activated carbon and stir for 30 minutes, filter out the activated carbon, and filter the liquid through 0.45 μm and 0.22 μm microporous membranes to detect the content of intermediates, calculated as cefpodoxime axetil 0.2g per bottle is calculated as filling volume.
[0053] 4) Fill according to ...
Embodiment 3
[0054] Example 3. Preparation of lyophilized powder injection of cefpodoxime axetil composition for injection (specification: 100 mg, calculated as cefpodoxime axetil), calculated in 1000 vials.
[0055] 1. Prescription:
[0056] Cefpodoxime Proxetil 100g
[0057] Chitosan Nanoparticles 40g
[0058] Water for injection 2000ml
[0059] 2. Preparation process:
[0060] 1) Slowly add the prescribed amount of chitosan nanoparticles into the prescribed amount of water for injection, and stir until dissolved while adding.
[0061] 2) Add the prescribed amount of cefpodoxime axetil and stir to dissolve until clear.
[0062] 3) Use sodium hydroxide to adjust the pH to 6.0, add 0.1% activated carbon and stir for 30 minutes, filter out the activated carbon, and filter the liquid through 0.45 μm and 0.22 μm microporous membranes to detect the content of intermediates, calculated as cefpodoxime axetil 0.2g per bottle is calculated as filling volume.
[0063] 4) Fill according to th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap