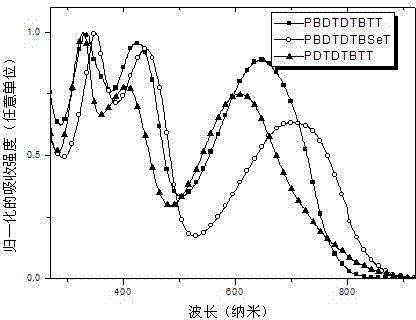
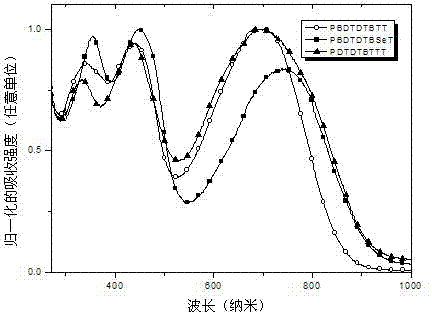
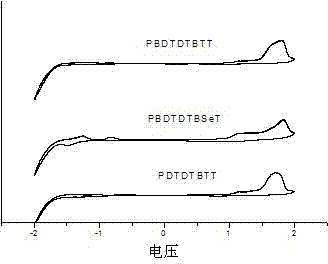
Thiophene condensed 2,1,3-benzoxadiazole derivative and polymer thereof
A technology of benzodiazole and derivatives, which is applied in the field of polymers and optoelectronic materials, can solve the problems of low photoelectric conversion efficiency of organic solar cells, and achieve good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: 4,8-bis-(5-bromothiophen-2 base)-2,1,3-benzothiadiazolo[4,5-b]thiophene-6-carboxylic acid-(2-ethane Synthesis of (ylhexyl) ester
[0045] The synthetic route is as follows:
[0046]
[0047] The synthesis method is:
[0048] Step 1: Preparation of N-(4-bromo-5-methyl-2-nitrophenyl)acetamide (2):
[0049]2-Nitro-5-methylaniline (1) (10.0 g, 65.7 mmol) and NBS (11.5 g, 64.6 mmol) were added to a 500 mL round bottom flask, and then 250 mL of glacial acetic acid was added. After the reactant was refluxed for 1.5 h, 10 ml of acetic anhydride was added thereto, and the reflux was continued for 4 h. After the reaction was completed, it was cooled to room temperature, and the mixture was poured into ice water to obtain a water-insoluble precipitate, which was filtered and washed three times with water, and the obtained product was After drying, 17.0 g of light yellow solid powder was obtained, with a yield of 99.7%. 1 H-NMR (300MHz, CDCl 3 ,δppm): 10.31(s,1H)...
Embodiment 2
[0062] Example 2: 4,8-bis-(5-bromothiophen-2 base)-2,1,3-benzoselenodiazolo[4,5-b]thiophene-6-carboxylic acid-(2-ethane Synthesis of (ylhexyl) ester
[0063] The synthetic route is as follows:
[0064]
[0065] The synthesis method is:
[0066] Step 1: Preparation of isooctyl 5-amino-6-nitrobenzo[4,5-b]thiophene-2-carboxylate:
[0067] Using 2-nitro-5-methylaniline as a raw material, 5-amino-6-nitrobenzo[4,5-b]thiophene-2-carboxylic acid isooctyl was synthesized according to the method of Example 1.
[0068] Step 2: Preparation of 4,8-dibromo-2,1,3-benzoselenodiazolo[4,5-b]thiophene-6-carboxylate-(2-ethylhexyl)ester (9):
[0069] In a 100 mL round bottom flask was added 5-amino-6-nitrobenzo[4,5-b]thiophene-2-carboxylate isooctyl (5) (0.40 g, 0.84 mmol) and SnCl 2 ﹒ 2H 2 O (0.48 g, 2.1 mmol), and then 10 mL of ethyl acetate was added. After reflux overnight, cool to room temperature, pour the reaction liquid into water, and extract and separate the liquids with ethyl ac...
Embodiment 3
[0074] Example 3: 4,8-bis-(5-bromothien-2yl)-2,1,3-benzothiadiazolo[4,5-b]thiophene-6-carboxylic acid-(2-butane Synthesis of octyl) esters
[0075] Synthetic route one:
[0076]
[0077] Synthetic route two:
[0078]
[0079] Synthetic route-corresponding method:
[0080] Step 1: Preparation of 5-amino-2-bromo-4-nitrobenzaldehyde:
[0081] Taking 2-nitro-5-methylaniline as raw material, synthesized according to the method of Example 1 to obtain 5-amino-2-bromo-4-nitrobenzaldehyde
[0082] Step 2: Preparation of ethyl 5-amino-6-nitrobenzo[4,5-b]thiophene-2-carboxylate (12):
[0083] Using 5-amino-2-bromo-4-nitrobenzaldehyde and ethyl thioglycolate as raw materials, synthesized according to the method of Example 1 to obtain 5-amino-6-nitrobenzo[4,5-b]thiophene- 2-Carboxylic acid ethyl ester, yield 99%. 1 H-NMR (300MHz, CDCl 3 ,δ): 8.67(s,1H), 7.81(s,1H), 7.25(s,1H), 5.84(s,2H), 4.42(q,2H), 1.42(t,3H).
[0084] Step 3: Preparation of ethyl 4,8-dibromo-2,1,3-benzothi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com