Method for continuously preparing epsilon-lactone
A caprolactone, continuous technology, applied in organic chemistry and other directions, can solve the problems of reducing production efficiency, occupying time, increasing control difficulty, etc., to improve production efficiency, reduce start-up and stop time, and reduce fluctuations in process parameters.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The cyclohexanone used in the preparation method of the present invention is preferably a compound with a purity of more than 99% by weight.
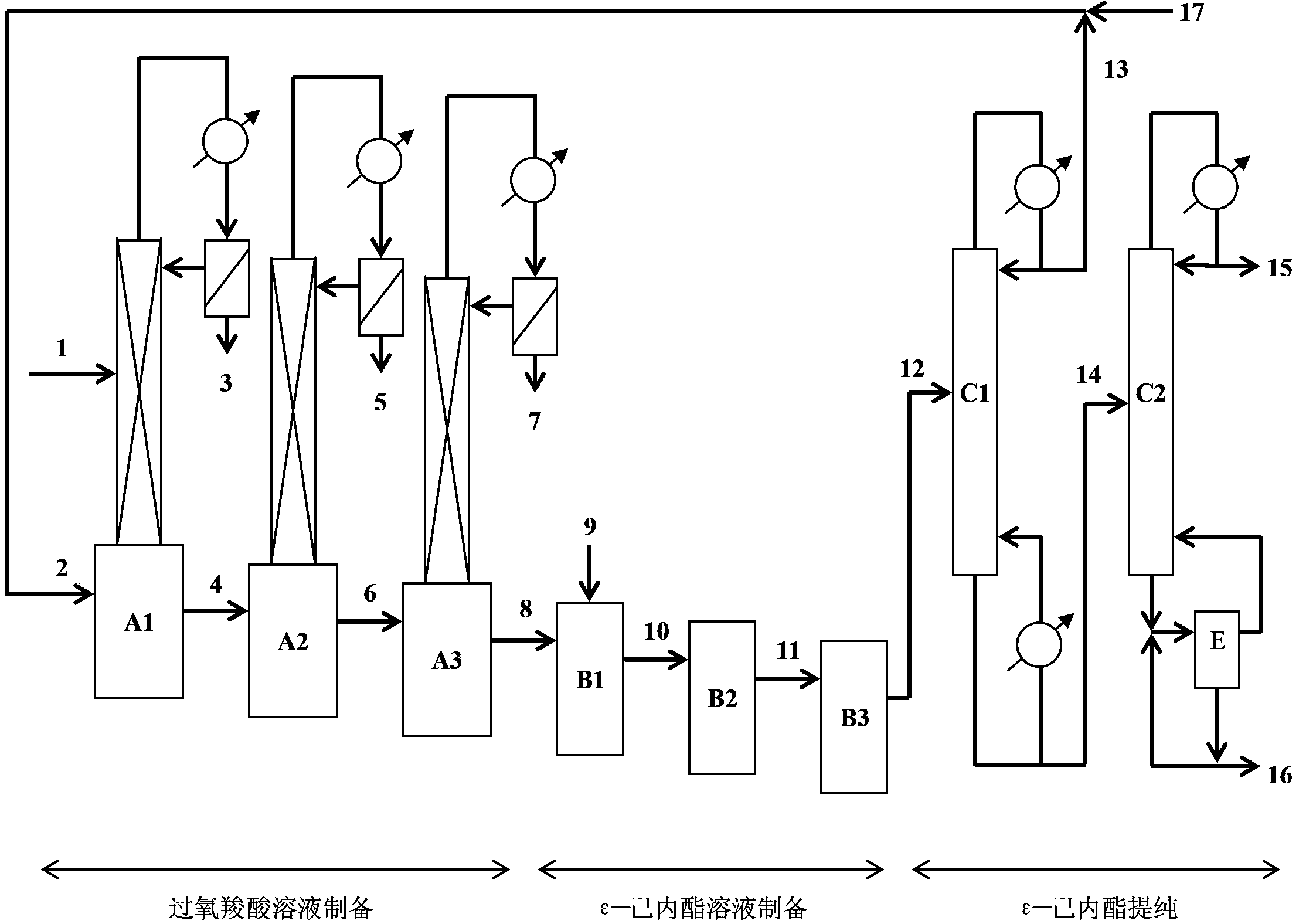
[0029] The content of ε-caprolactone in the obtained crude ε-caprolactone solution is preferably 10-50%, the rest is carboxylic acid and organic solvent, the content of peroxycarboxylic acid is less than 3%, and the content of cyclohexanone is less than 0.7%. .
[0030] The purification step of separating ε-caprolactone from the crude ε-caprolactone solution can follow the usual distillation method, for example, the ε-caprolactone solution is rectified through two rectification towers, and the ε-caprolactone solution Enter tower C1, distill organic solvent and carboxylic acid from the top of the tower, the liquid from the bottom of the tower enters the tower C2, distill high-purity caprolactone from the top of the tower, and discharge the liquid containing high boiling point impurities and boric acid from the bottom of the tower....
Embodiment 1
[0037] In this example, the acetic acid / ethyl acetate system was adopted, and two kettles were connected in series for the preparation of peracetic acid and ε-caprolactone, and the total residence time was 2 hours. The composition of the reaction solution is: 20% acetic acid, 79.8% ethyl acetate, and 0.2% boric acid.
[0038] Preparation of peracetic acid solution
[0039] Use a pump to send the reaction solution from pipe 2 into A1 at a rate of 595 g / h. At the same time, use a pump to send hydrogen peroxide with a content of 50% from pipe 1 to the middle of A1 tower at a speed of 119g / h, adjust the system pressure to 40~50kPa, control the temperature of the liquid in the kettle to 65~80°C, and maintain boiling in the kettle. It rises to the top of the tower through the rectification column, and after being condensed, it is separated in the water separator. The lower aqueous phase is continuously discharged through the pipe 3, and the upper organic phase is continuously refluxe...
Embodiment 2
[0048] In this example, the propionic acid / ethyl acetate system was adopted, and the preparation of peroxypropionic acid and ε-caprolactone were both carried out in series with three kettles, and the total residence time was 4.5 h. The composition of the reaction liquid is: propionic acid 40%, ethyl acetate 59.6%, boric acid 0.4%.
[0049] Preparation of peroxypropionic acid solution
[0050] Use a pump to send the reaction solution from pipe 2 into A1 at a rate of 440 g / h. At the same time, use a pump to send hydrogen peroxide with a content of 50% from pipe 1 to the middle of A1 tower at a speed of 68g / h, adjust the system pressure to 15~25kPa, control the temperature of the liquid in the kettle to 60~70°C, and maintain boiling in the kettle. It rises to the top of the tower through the rectification column, and after being condensed, it is separated in the water separator. The lower aqueous phase is continuously discharged through the pipe 3, and the upper organic phase is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com