Bay-site cyclization synthetic method of 3, 4:9, 10-perylenetetracarboxylic bisimide
A perylenetetracarboxylic acid diimide and a synthesis method technology are applied in the synthesis field of the cyclic reaction of the basal position, which can solve the problems of poor solubility and lower synthesis value, and achieve the avoidance of perylene plane distortion, simple reaction steps, Enriching the effect of derivatization methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
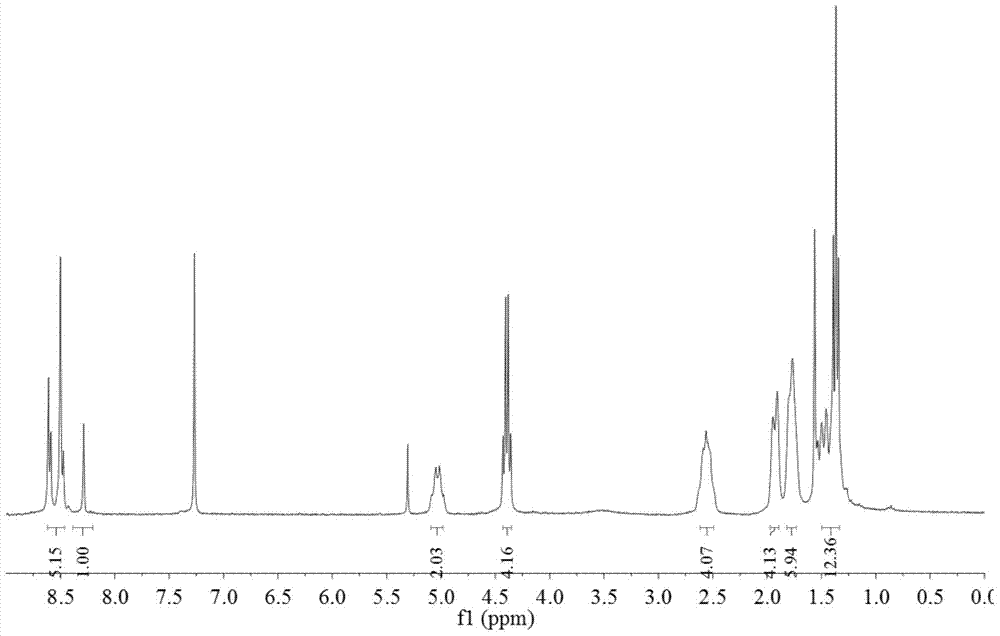
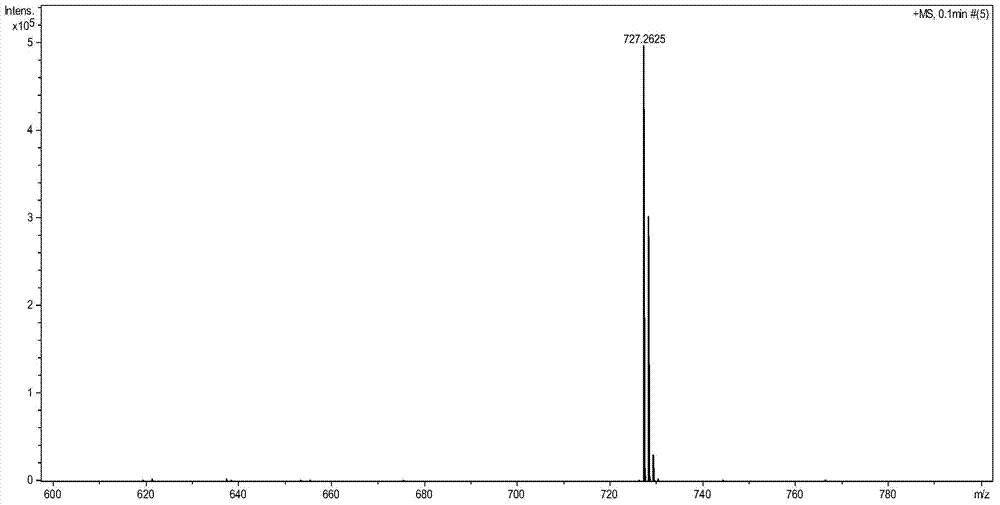
[0031] Take 600 mg of N,N'-dicyclohexyl-1-nitro-3,4:9,10-perylenetetracarboxylic acid diimide, 0.35 ml of diethyl malonate and 415 mg of potassium carbonate dissolved in 30 Milliliter of N-methylpyrrolidone, stirred at room temperature, reacted for 3 hours, the reaction solution was dropped into 100 ml of 2mol / L dilute hydrochloric acid, precipitated, suction filtered, washed 3 times with water, and dried. The crude product was subjected to silica gel column chromatography, and the eluent was dichloromethane:ethyl acetate=20:1. Obtain product A (structure sees attached figure 1 , 2 shown) 580 mg, 80% yield.
Embodiment 2
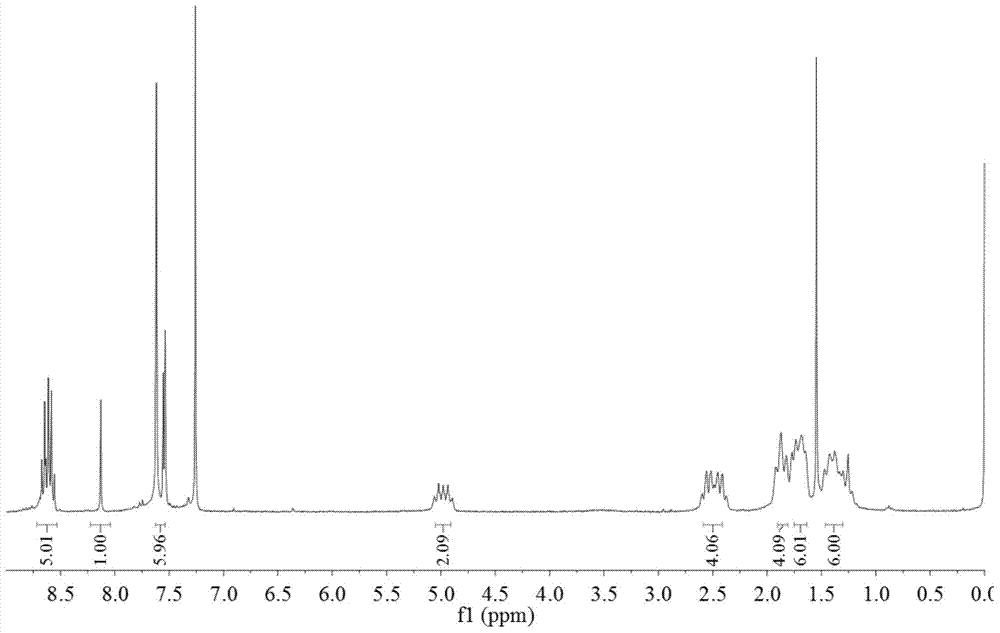
[0033] Take 600 mg of N,N'-dicyclohexyl-1-nitro-3,4:9,10-perylenetetracarboxylic acid diimide, 650 mg of 2,7-dibromofluorene and 415 mg of sodium carbonate in 30 ml of dioxane was reacted for 5 hours, cooled, and the reaction solution was dropped into 100 ml of 2mol / L dilute hydrochloric acid to precipitate a precipitate, filtered with suction, washed with water 3 times, and dried. The crude product was subjected to silica gel column chromatography, and the eluent was dichloromethane. Obtain product B (structure sees attached image 3 , 4 shown) 490 mg, yield 55%.
Embodiment 3
[0035] Take 645 mg of N,N'-dicyclohexyl-1,6-dinitro-3,4:9,10-perylenetetracarboxylic acid diimide, 0.4 ml of 2-nitropropane and 830 mg of potassium acetate In 30ml of N,N'-dimethylacetamide, heat to 60°C, react for 2.5 hours, cool down, drop the reaction liquid into 100ml of 2mol / L dilute hydrochloric acid, precipitate out, filter with suction, wash with water 3 times ,drying. The crude product was subjected to silica gel column chromatography, and the eluent was dichloromethane. Obtain product C (structure sees attached Figure 5 , 6 shown) 540 mg, yield 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com