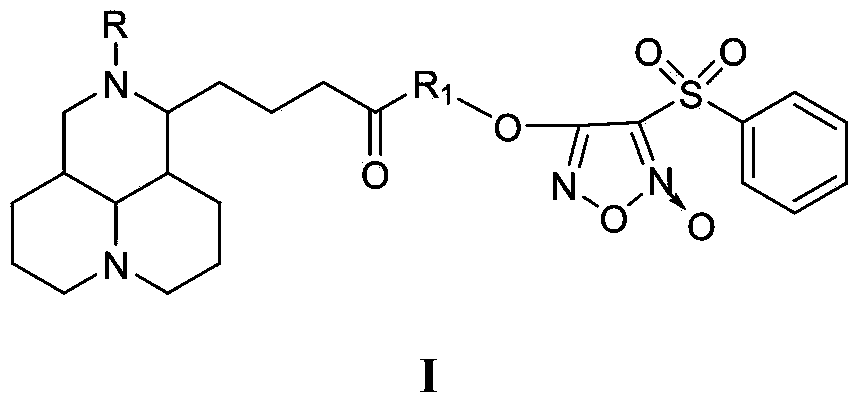
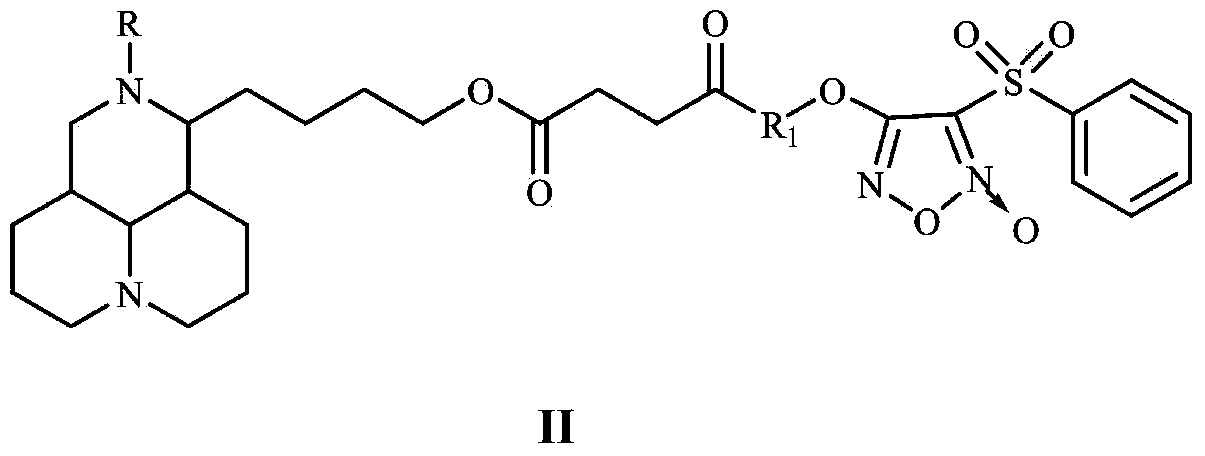
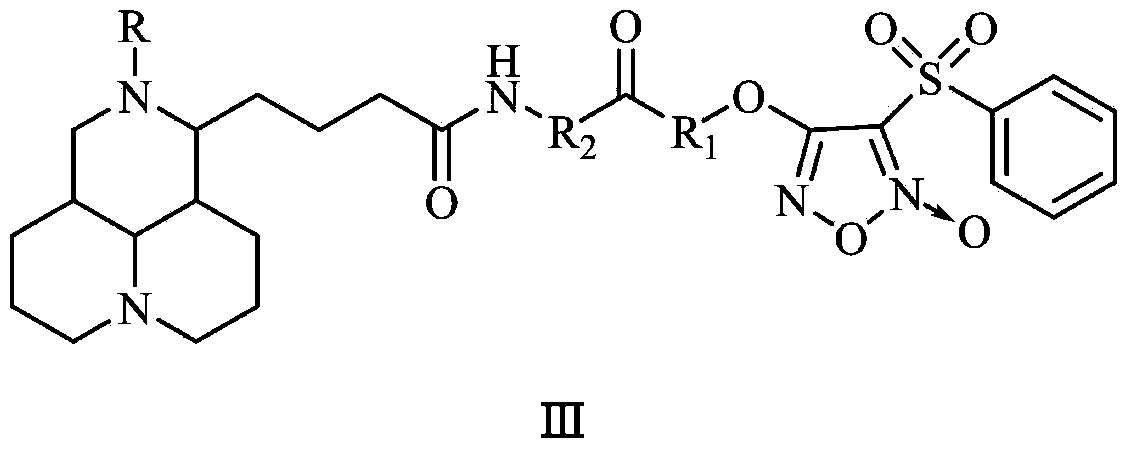
NO donor type matrine derivative and preparation method and medical application thereof
A technology of matrine and derivatives, applied in the fields of medicinal chemistry and pharmacotherapy, can solve the problems of weak, wide pharmacological effects, limited development and utilization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0229] Synthesis of N-n-butylmatrine (4)
[0230] Take matrine (4.96g, 0.02moL) in a 250mL round bottom flask, add 10% sodium hydroxide (80mL, 0.2moL), reflux reaction under stirring, TLC detection of reaction progress. After the reaction is complete, adjust the pH to 7-8 with 20% sulfuric acid solution under ice bath. Concentrate under reduced pressure, dissolve the residue by heating with methanol, and filter while hot. The filtrate was concentrated under reduced pressure to 1 / 4 volume, acetone was added, stirred vigorously, a precipitate was precipitated, and filtered by suction to obtain 4.06 g of matrine as a white solid. Take matrine acid (2.66g, 0.01mol) and anhydrous K2CO3 (6.9g, 0.05mol) and dissolve it in 20ml of DMF, condense and reflux under stirring at 60-70°C, when the temperature rises to 60°C and the raw materials are completely dissolved, add 3.5 ml (0.03mol) n-butane bromide, which lasted about 6 hours, TLC detected that the reaction was complete. Suction ...
Embodiment 2
[0239] Referring to the method of Example 1, N-n-butyl matrine and 3,4-diphenylsulfonyl-1,2,5-oxadiazole-2-oxide were prepared.
[0240] Preparation of Aminoethanol Furazan (5)
[0241] Add NaH (142mg, 5.9mmol) and aminoethanol (0.2ml, 3.33mmol) into a 50ml round-bottomed flask, keep away from light, stir in an ice-salt bath, and add furazan dissolved in THF, that is, 3,4-dibenzenesulfonate Acyl-1,2,5-oxadiazole-2-oxide (0.5g, 1.37mmol), keep the reaction on ice. TLC detected that the reaction was complete. Water was added to the reaction solution, and after extraction with EtOAc, the organic layer was washed with saturated NaCl, and TLC detected that the organic layer had a single spot. Anhydrous Na 2 SO 4 After drying, it was concentrated to dryness under reduced pressure and weighed 239 mg. The yield is 62%.
[0242] N-Butyl matrine aminoethanol furazan (I 11 )Synthesis
[0243] Add N-n-butyl matrine (85mg, 0.264mmol), DCC (54.4mg, 0.264mmol) into a 50ml round bott...
Embodiment 3
[0245] Referring to Example 1, benzyl N-benzyl matrine and 1,2-ethylene glycol furazan were synthesized.
[0246] Preparation of N-benzylmatrine succinate (6)
[0247] Add 4.48g (0.01mol) benzyl N-benzyl matrine and 35mlTHF into a 100ml round bottom flask, slowly add 0.68g lithium tetrahydrogen aluminum under ice bath conditions, stir for 2 hours, TLC detection shows that the reaction is complete, stop the reaction , slowly add water dropwise under stirring until no bubbles are produced. Suction filtration, the filtrate was extracted with ethyl acetate (3 x 30ml). The organic layer was washed twice with water, anhydrous Na 2 SO 4 Dry and filter. The filtrate was concentrated to dryness under reduced pressure to obtain 3.04 g of milky white powder, namely N-benzylmatrine. mp: 80.0-80.2°C, yield: 88.9%. ESI-MS: 343[M+H] + ;IR(KBr,cm -1 ):υ:3426(O-H);3030(Ar-H);2932,2818(C-H);1612,1558(C 6 h 6 ); 1 H-NMR (300MHz, CDCl 3 ),δ(ppm):1.47-1.97(m,18H,CH 2 ,CH);2.03(s,1H,CH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com