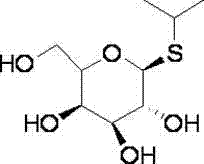
Method for preparing isopropyl-beta-D-thiogalactoside
A technology of thiogalactoside and propylthioacetyl, which is applied in the field of preparation of isopropyl-β-D-thiogalactoside, which achieves the effect of simple operation, easy availability of raw materials, and improved safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The post-treatment in step a is to dropwise add 10 mol of water at 0-5°C and stir for 2 hours, extract the aqueous layer with 5 mol of dichloromethane, separate the organic phase and wash the organic phase with 10 mol of water three times, separate the organic phase and concentrate, add 1mol tert-butyl methyl ether and 2mol isohexane mixed solution crystallized, filtered and dried.
[0026] The post-treatment in step b is to concentrate to dryness, add 0.2 mol ethanol and 1.8 mol tert-butyl methyl ether for crystallization, filter and dry.
[0027] Example 2 5.5 : 1.5 : 1: 1.2
[0028] a) Add 5.5 mol of acetic anhydride and 1.5 mol of aluminum trichloride at room temperature, add 1 mol of galactose in 12 batches at 5-10 °C, after the reaction is completed, add 1.2 mol of isopropyl mercaptan, after the reaction is completed, 0.771 mol is obtained after post-treatment Isopropylthioacetylgalactose;
[0029] b) Add 0.771 mol of isopropylthioacetylgalactose to 10 mol of me...
Embodiment 2
[0030] The post-treatment in step a is to dropwise add 10 mol of water at 0-5°C and stir for 2 hours, extract the aqueous layer with 5 mol of dichloromethane, separate the organic phase and wash the organic phase with 10 mol of water three times, separate the organic phase and concentrate, add 1mol tert-butyl methyl ether and 2mol isohexane mixed solution crystallized, filtered and dried.
[0031] The post-treatment in step b is to concentrate to dryness, add 0.2 mol ethanol and 1.8 mol tert-butyl methyl ether for crystallization, filter and dry.
[0032] Example 3 6:1.5: 1: 1.2
[0033] a) Add 6 mol of acetic anhydride and 1.5 mol of aluminum trichloride at room temperature, add 1 mol of galactose in 12 batches at 5-10°C, add 1.3 mol of isopropyl mercaptan after the reaction is completed, and obtain 0.774 mol of isopropyl mercaptan after the reaction is completed Propylthioacetylgalactose;
[0034] b) Add 0.774 mol of isopropylthioacetylgalactose to 10 mol of methanol to di...
Embodiment 3
[0035] The post-treatment in step a is to dropwise add 10 mol of water at 0-5°C and stir for 2 hours, extract the aqueous layer with 5 mol of dichloromethane, separate the organic phase and wash the organic phase with 10 mol of water three times, separate the organic phase and concentrate, add 1mol tert-butyl methyl ether and 2mol isohexane mixed solution crystallized, filtered and dried.
[0036] The post-treatment in step b is to concentrate to dryness, add 0.2 mol ethanol and 1.8 mol tert-butyl methyl ether for crystallization, filter and dry.
[0037] Example 4 6 : 1 : 1: 1.1
[0038] a) Add 6mol acetic anhydride and 1mol zinc chloride at room temperature, add 1mol galactose in 12 batches at 5-10°C, add 1.1mol isopropyl mercaptan after the reaction is completed, and obtain 0.732mol isopropyl after the reaction is completed Thioacetylgalactose;
[0039] b) Add 0.732 mol of isopropylthioacetylgalactose to 10 mol of methanol to dissolve, add 0.01 mol of sodium methoxide, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com