Use of low dose Il-2 for treating autoimmune - related or inflammatory disorders
An autoimmune disease and immune-related technology, which is applied in the application field of low-dose IL-2 for the treatment of autoimmune-related diseases or inflammatory diseases, and can solve problems such as exacerbation of diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
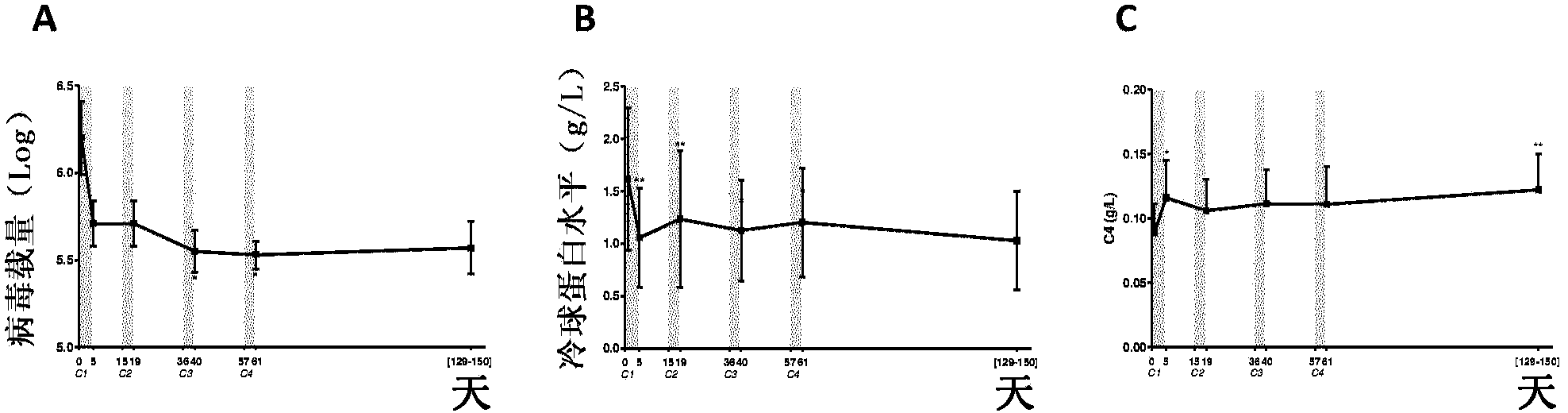
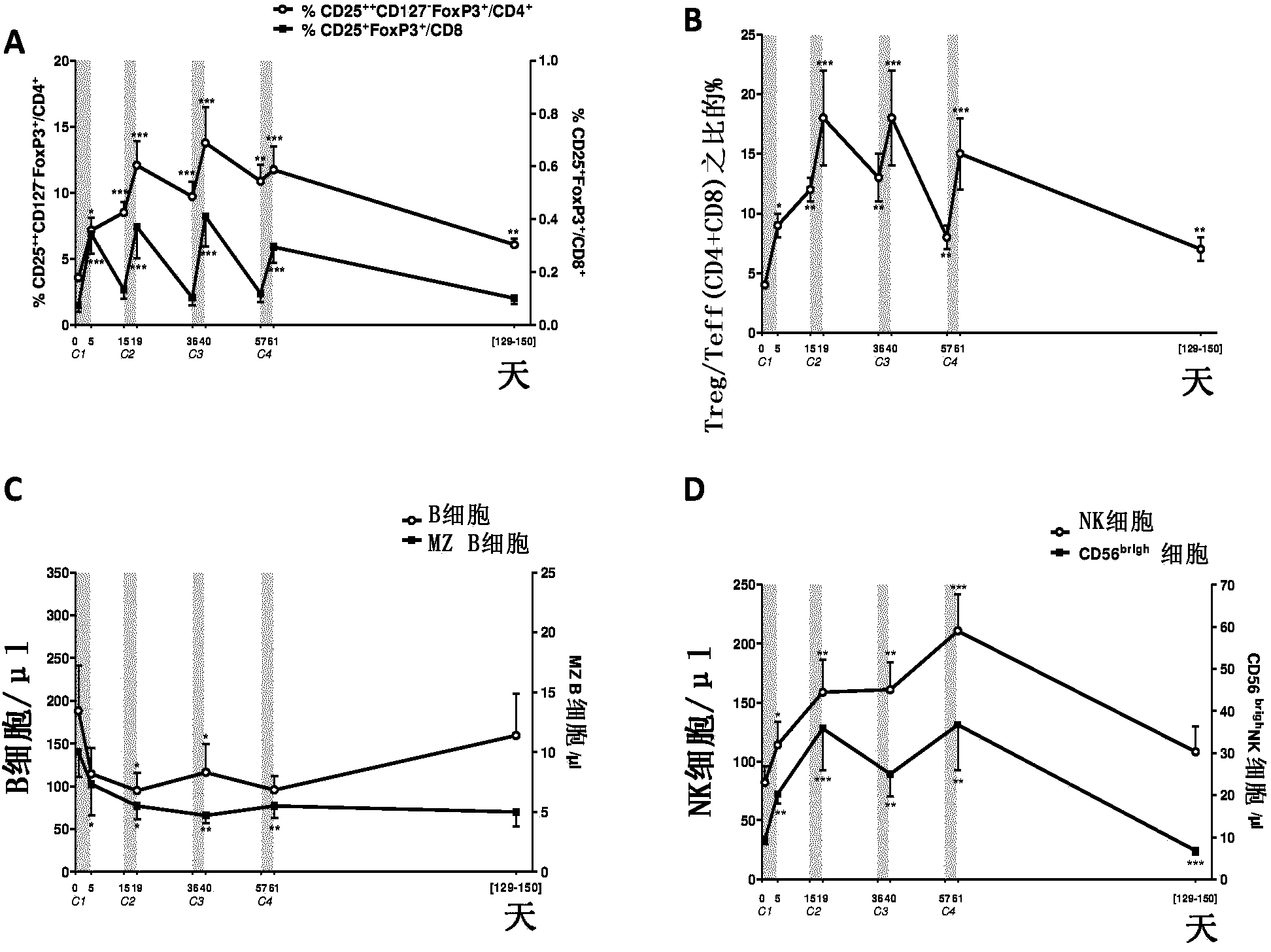
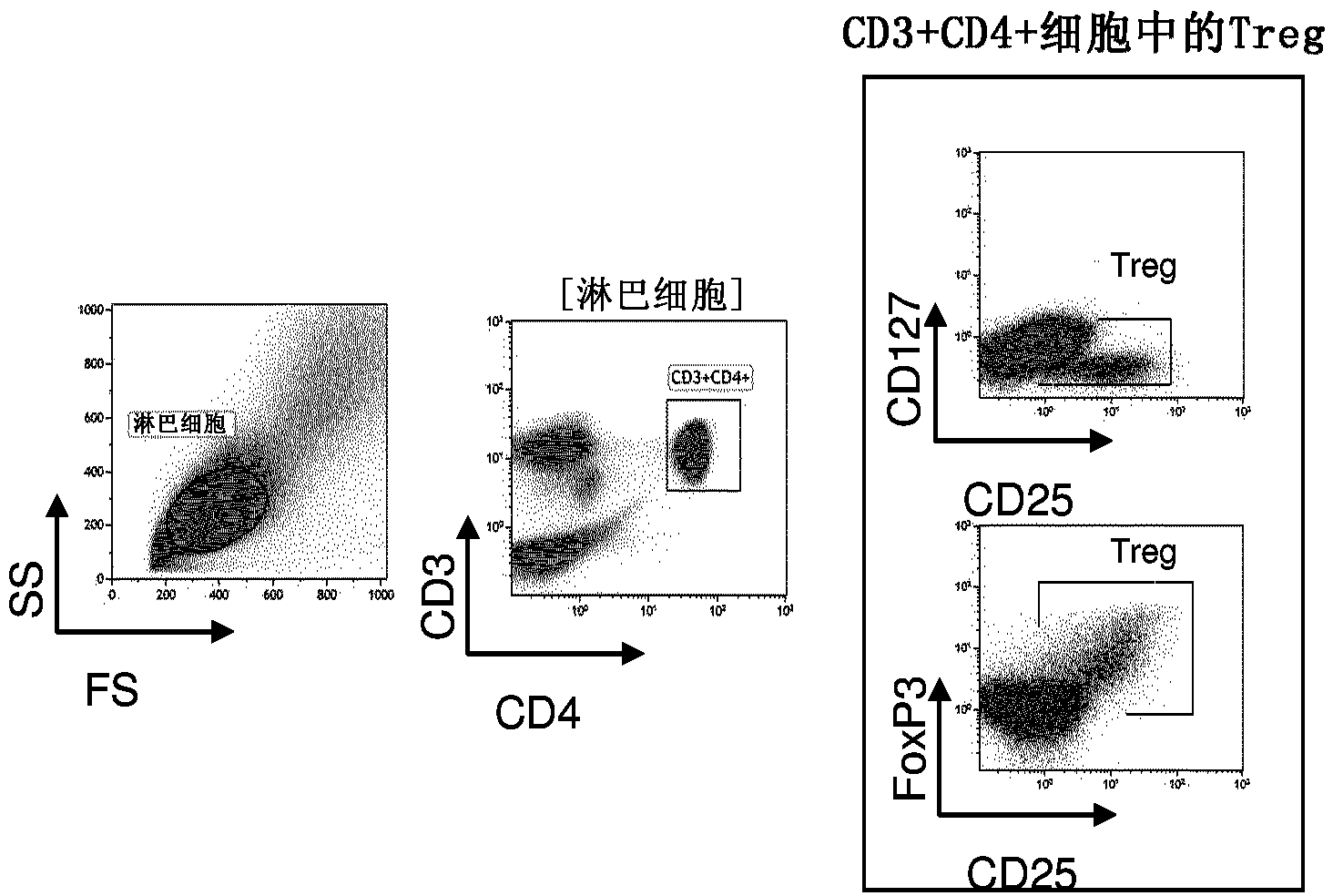
[0223] Example 1: Low Dose IL-2 in HCV-Associated Vasculitis
[0224] Here, we provide the first evidence in human subjects that IL-2 can be used to induce Tregs without inducing Teffs in patients with autoimmunity. Here we report the first demonstration of in vivo expansion of very potent suppressive Tregs by IL-2 immunotherapy in human autoimmune diseases, leading to clinical improvement. The primary endpoint of our study, increased Treg at the end of IL-2 therapy, was met as well as all secondary priorities, including clinical response. We show that low-dose IL-2 is well tolerated, induces a sharp and selective increase in Treg cells, and leads to clinical improvement in 80% of patients. This is the first demonstration of Treg induction and recovery in vivo following IL-2 immunotherapy in a human autoimmune disease. Furthermore, we show for the first time a significant anti-inflammatory effect of low-dose IL-2 in humans.
[0225] method
[0226] patient
[0227] Incl...
Embodiment 2
[0272] Example 2: Low Dose IL-2 in Type 1 Diabetes Mellitus
[0273] The present inventors initiated an IL-2 dose-finding clinical trial in T1D, which aimed to determine the lowest active dose capable of safely inducing Tregs in adult T1D patients.
[0274] The DF-IL2 trial was double-blind and compared placebo, 0.3, 1 and 3mIU / day Dose (cumulative doses were 1.5, 5 and 15mIU).
[0275] The aim of the trial was to preserve residual endogenous insulin secretion in newly diagnosed T1D patients.
[0276] Key patient characteristics: adult, both sexes, T1D diagnosis per WHO-ADA, disease duration less than 12 weeks from diagnosis at first IL-2 dose, and C-peptide available at entry detection.
[0277] To achieve a clinically meaningful effect, the currently recommended goal is to preserve at least the pancreatic β-cell mass with active therapy, ie maintain an AUC of C-peptide relative to baseline of 0-120.
[0278] All 24 patients were included. Although investigators remain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com