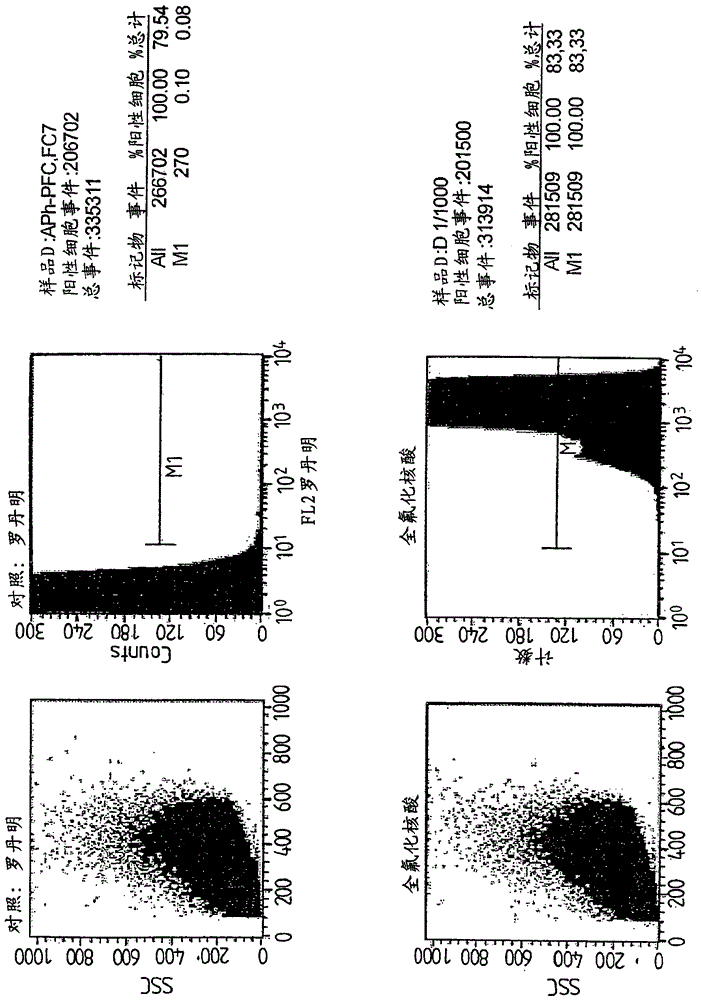
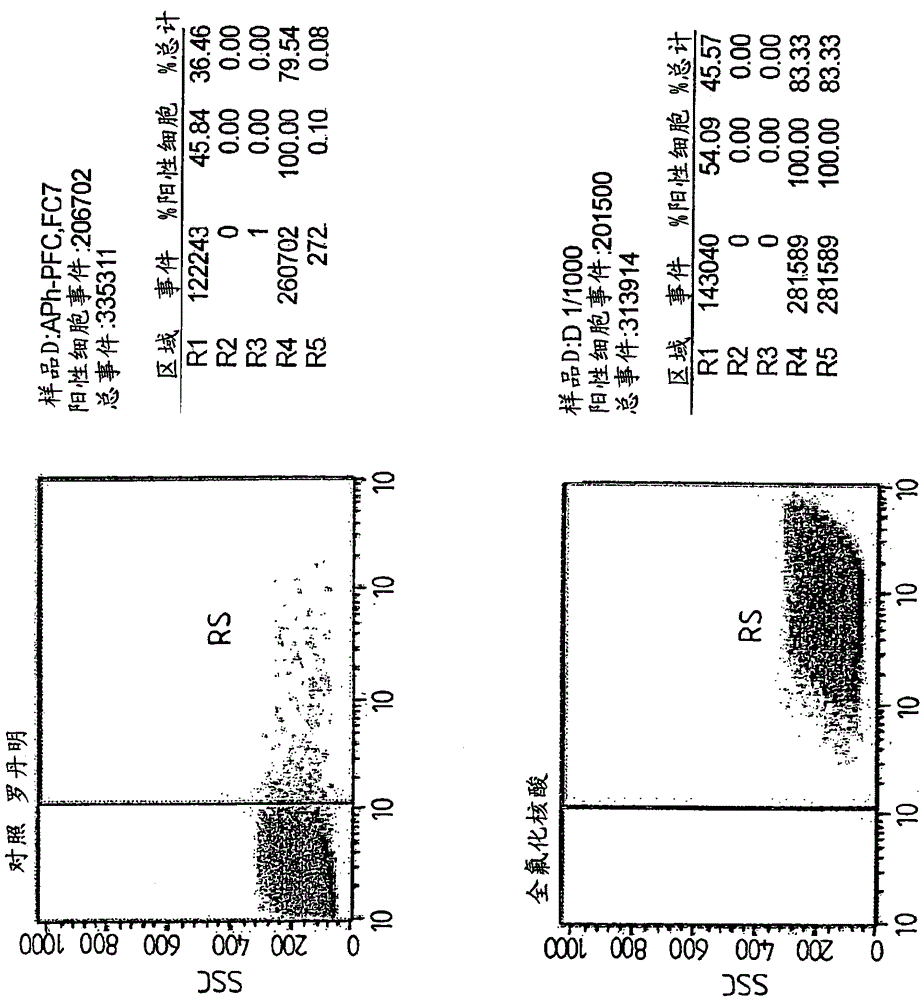
Perfluorinated compounds for non-viral transfer of nucleic acids
A compound and perfluorocarbon technology, which is applied in the direction of introducing foreign genetic material, drug combination, and medical preparations with non-active ingredients by using carriers, etc., can solve problems such as adverse effects, low efficiency, and inability to achieve viral gene transfer efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0177] Sample Embodiment 1: Synthesis of Perfluorinated Nucleobases
[0178] Perfluorination of nucleobases was performed by Williamson ether synthesis. Here, NH 2 groups are relatively easy to reach. Thymine does not have NH 2 group, but six tautomeric structures can occur, four of which have perfluorinatable OH groups.
[0179]
Embodiment approach 2
[0180] Sample embodiment 2: Synthesis of 2'-perfluorinated nucleosides based on 2'perfluorinated uridines
[0181] Uridine is an important component of RNA. Insertion into the RNA chain via the 3' or 5' OH group of the sugar. The 2' position of uridine is therefore particularly suitable for introducing substituents in the case of nucleobase bonding sites. A variety of 2'-substituted uridines are known with attachment via 2' ethers or esters, 2' thioethers or esters, 2' amides or 2' carbamates or even via 2-C; see Scheme 8.
[0182]
[0183] Scheme 8: Examples of 2-substituted uridines suitable for perfluorination at the 2' position
[0184] In order to synthesize uridines with perfluorinated alkenes at the 2' position, a new synthetic method was developed. For example, direct attachment to the 2'-OH group via ether functionality and ester functionality; see Scheme 9.
[0185]
[0186] Scheme 9: Direct attachment to the 2'OH group via ether and ester functions
[018...
Embodiment approach 3
[0199] Sample embodiment 3: Perfluorination via the 2'-amino functional group of 2'-amino-2'-deoxyuridine
[0200] The second synthetic route starts with 2'-amino-2'-deoxyuridine, which is commercially available. This is converted to amides using perfluorinated carboxylic acids. Here, an inert gas is used with the exclusion of moisture. The final product was purified using preparative column chromatography. The primary OH group at the 5' position of uridine is protected by DMT. Appropriate protecting groups may be added or omitted for similar reactions at other positions of the nucleic acid component; see Scheme 13.
[0201]
[0202] Diagram 13
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com