Process for forming an aramid copolymer
A technology of polymers and oligomers, which is applied in the field of preparing aromatic polyamide polymers, and can solve problems such as the control of the position of monomer components that do not have
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0062] NMP, DMAC, LiCl, CaCl 2 , DAPBI, PPD and TCl were obtained from commercial sources.
example 1
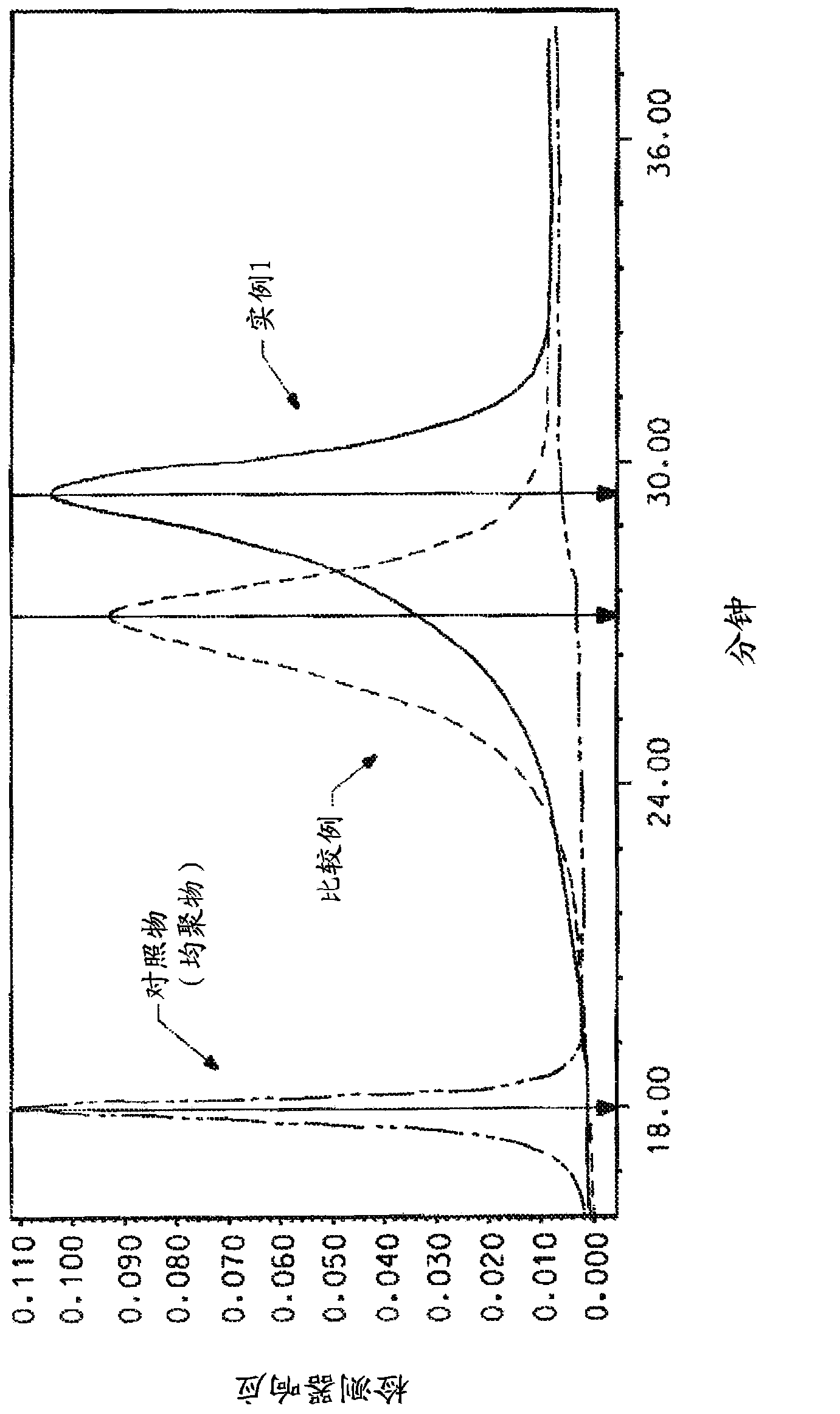
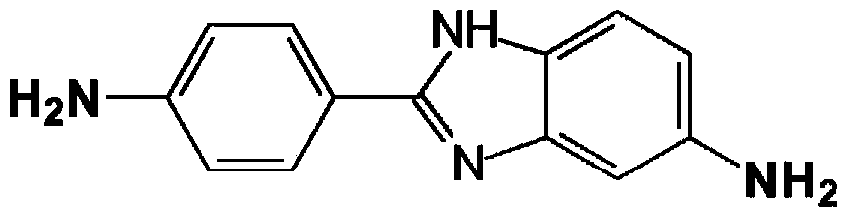
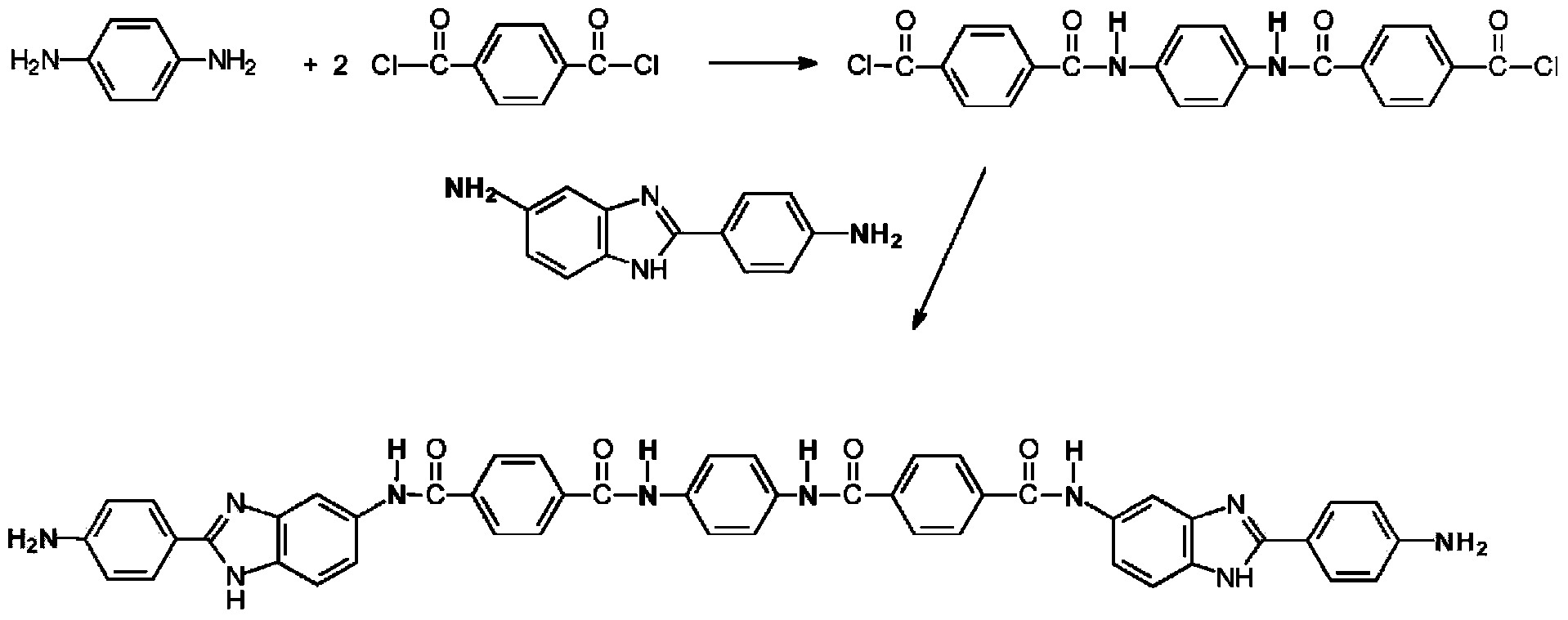
[0064] Add 104.64 g of NMP / CaCl to a 1 L reactor equipped with frame stirrer, nitrogen inlet / outlet 2 Premix (8.3% by weight (salt weight / total weight of salt and solvent)), 177.09 grams of NMP (N-methyl-2-pyrrolidone), and 2.539 grams (0.023 moles) of PPD (p-phenylenediamine), and Stir for 10 minutes. The contents were stirred in an ice-water bath to cool the mixture to below 10°C. Add 9.536 g (0.047 mol) TCl in one portion and stir for 5 minutes. The ice water bath was removed and 12.288 grams (0.055 moles) of DAPBI were added and stirred. The solution became very viscous and gelled within about 4 minutes. The highly viscous reaction mixture was stirred for an additional 25 minutes. Transfer the resulting polymer to blender and ground into small particles, then washed several times to remove solvent (NMP / CaCl 2 ) and excess HCl produced by the reaction. The polymer was then neutralized with sodium bicarbonate and finally washed several times with water to obtain a ne...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com