Application of the substance reducing the combination of β-arrestin 1 and aph-1 protein in the preparation of drugs for preventing and treating neurodegenerative diseases
A neurodegenerative and inhibitory protein technology, applied in the direction of nervous system diseases, peptide/protein components, applications, etc., can solve problems such as the unclear role of β-Arrestin1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0237] Example 1. Increased expression levels of β-Arrestin in Alzheimer's disease humans and transgenic model mice
[0238] First, in order to investigate whether β-Arrestin is altered in Alzheimer's disease, the inventors detected the expression level of β-Arrestin in brain samples of patients. Patient samples were evaluated according to the Braak staging method dependent on neuropathological indicators, and were divided into 7 groups according to the degree of lesion (Braak 0, I, II, III, IV and VI stages in turn) [Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta. Neuropathol. 82(4), 239-259 (1991)]. like figure 1 and figure 2As shown, the expression of β-Arrestin was detected by Western hybridization, and it was found that the protein level of β-Arrestin1 was positively correlated with Braak stage (r=0.725, Pfigure 1 and image 3 As shown, the expression level of β-Arrestin2 was also positively correlated with Braak stage (r=0.508, P<...
Embodiment 2
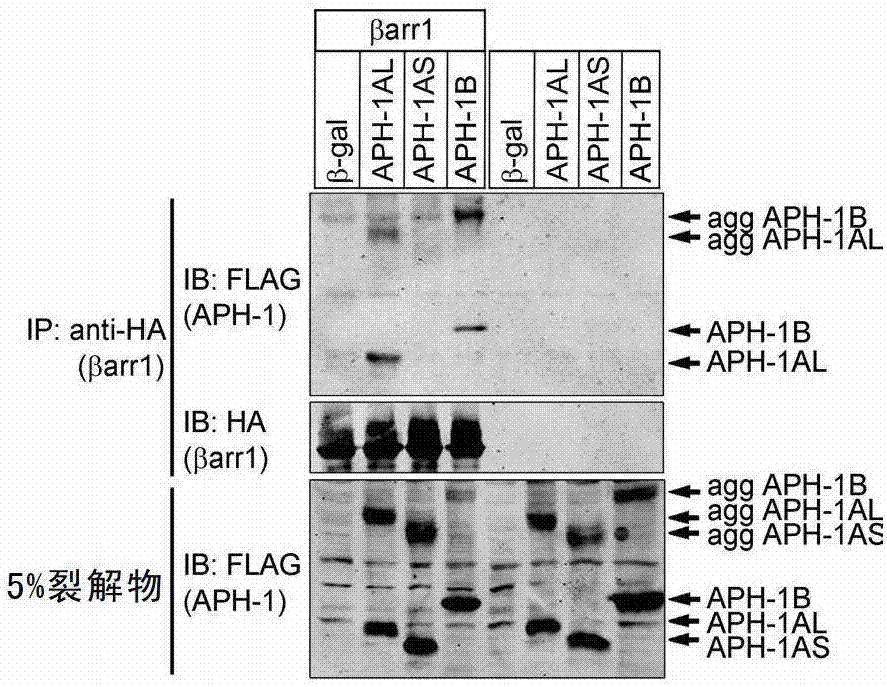
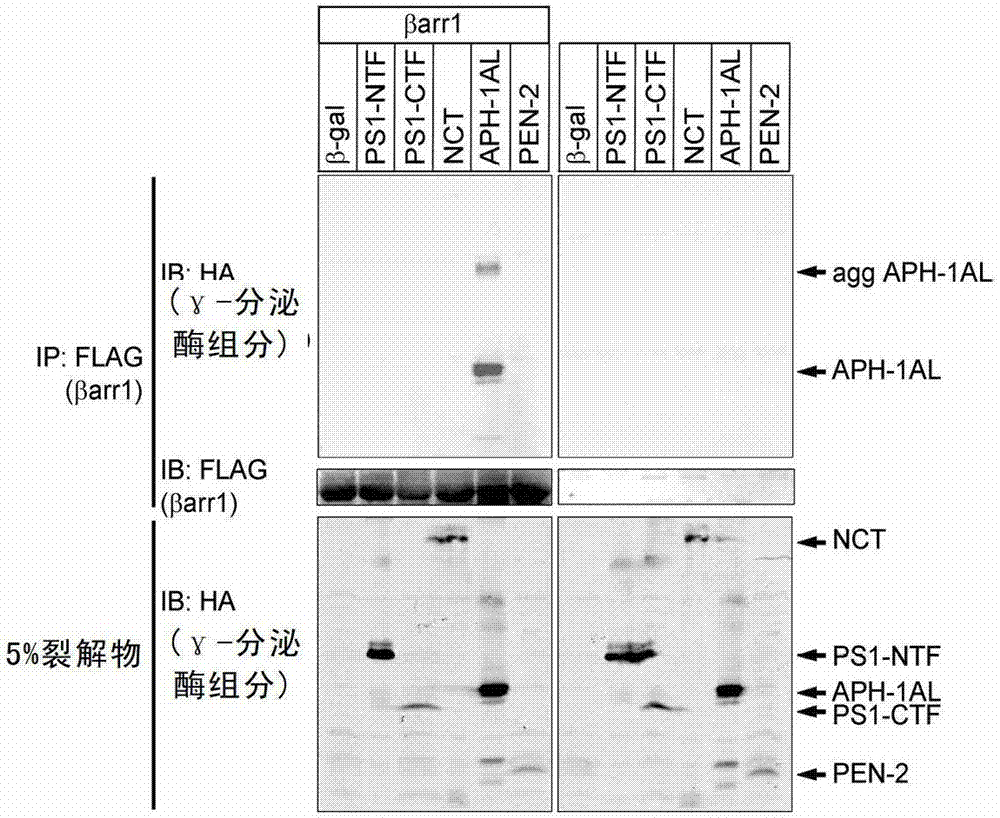
[0252] Example 2. β-Arrestin1 regulates γ-secretase activity by binding to APH-1AL
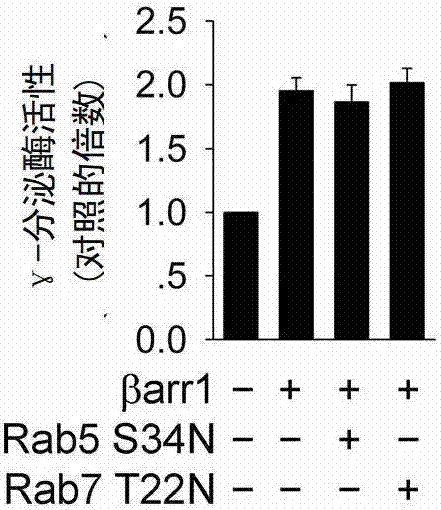
[0253] The inventor's previous research found that activated β2AR can enhance its protease activity by mediating γ-secretase into the endocytic pathway. In order to test whether endocytosis is involved in the regulation of β-Arrestin1 on γ-secretase, the inventors used dominant negative mutants of Rab5 and Rab7 (Rab5S34N and Rab7T22N) that regulate the formation of endocytic transport vesicles to inhibit endocytosis translocation process, and found that neither Rab5S34N nor Rab7T22N could inhibit the γ-secretase activity enhanced by β-Arrestin1 ( Figure 17 ). Therefore, the possibility that endocytosis is involved in the regulation of β-Arrestin1 on γ-secretase is ruled out.
[0254] β-Arrestin1 can regulate different signaling pathways by binding to cell surface receptors and intracellular signaling pathway molecules. The inventors speculate that protein-protein interaction may be involve...
Embodiment 3
[0264] Example 3. β-Arrestin1 regulates the assembly of γ-secretase complex
[0265] APH-1AL is a scaffold protein in the assembly process of the γ-secretase complex, which is necessary for the formation of a stable and active γ-secretase multiprotein large molecular weight complex [Niimura, M. et al. Aph- 1 contributes to the stabilization and trafficking of the γ-secretase complex through mechanisms involving intermolecular and intramolecular interactions. J. Biol. Chem. 280(13), 12967-12975(2005)]. APH-1AL deletion or mutation can lead to the inability to assemble and activate γ-secretase [Lee, S.F. et al. A conserved GXXXG motif in APH-1 iscritical for assembly and activity of the γ-secretase complex. (6), 4144-4152 (2004)]. In order to test whether β-Arrestin1 affects the assembly and activation process of γ-secretase, the inventors used glycerol gradient centrifugation to separate the γ-secretase complex from mouse hippocampus tissue extracts, and analyzed the molecular...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com