Ferrous carbonate/graphene composite material and preparation method and applications thereof
A technology of ferrous carbonate and composite materials, applied in the direction of active material electrodes, structural parts, electrical components, etc., can solve the problems of capacity decay, particle separation and rupture, and achieve the effect of high specific capacity, low cost, and easy control of process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Step 1: Weigh 0.020 g of graphene oxide and add it into a beaker filled with 40 mL of deionized water, mix well, and then prepare graphene oxide dispersion A through ultrasonic dispersion. Then, ferrous sulfate heptahydrate was added into the dispersion A, stirred and dissolved to obtain a dispersion B of ferrous sulfate, graphene and water. Wherein the mass ratio of graphene oxide to ferrous sulfate is 0.09:1.
[0039] Then, according to the concentration ratio of urea and ferrous sulfate as 30:1, add urea into the dispersion B, stir to completely dissolve the urea, and obtain the suspension C.
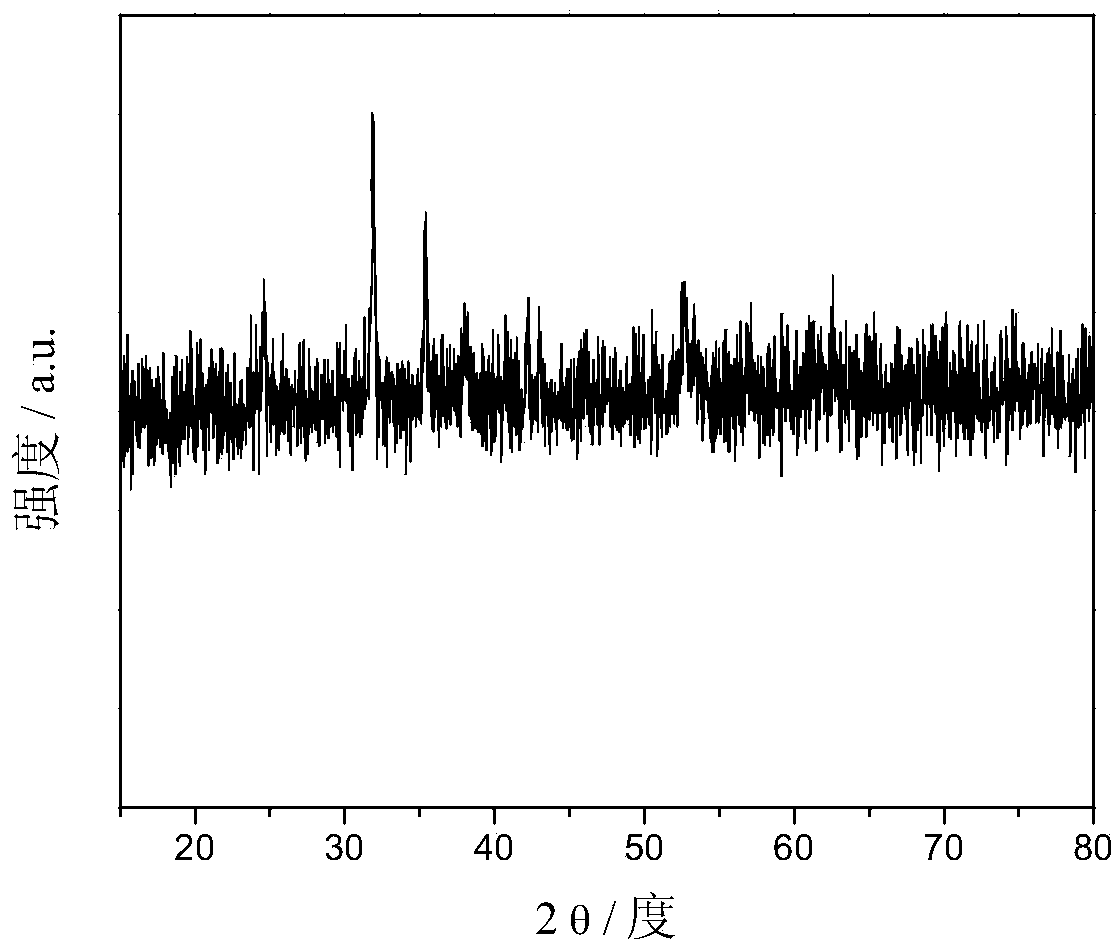
[0040] Step 2: Transfer the suspension C to a polytetrafluoroethylene-lined stainless steel reaction kettle, and place it in a blast oven at 120° C. for 8 hours. The obtained reaction product was repeatedly washed with deionized water and ethanol to obtain a ferrous carbonate / graphene composite material. figure 1 The XRD pattern of the powder of the ferrous carbonate / graphen...
Embodiment 2
[0047] Step 1: Weigh 0.040g of graphene oxide and add it into 40mL of deionized water, mix well, and prepare graphene oxide dispersion A through ultrasonic dispersion. Then, ferrous sulfate heptahydrate was added into the dispersion A, stirred and dissolved to obtain a dispersion B of ferrous sulfate, graphene and water. Wherein the mass ratio of graphene oxide to ferrous sulfate is 0.18:1.
[0048] Then, according to the concentration ratio of urea and ferrous sulfate as 100:1, add urea into the dispersion B, stir to completely dissolve the urea, and obtain the suspension C.
[0049] Step 2: Transfer the suspension C to a polytetrafluoroethylene-lined stainless steel reactor, and then place the reactor in a blast oven at 180° C. for 12 hours. The obtained reaction product was repeatedly washed with deionized water and ethanol to obtain a ferrous carbonate / graphene composite material, wherein the mass ratio of ferrous carbonate to graphene was 1.375:1. Figure 5 The TEM imag...
Embodiment 3
[0052] Step 1: Weigh 0.005g of graphene oxide and add 40mL of deionized water to mix, and prepare graphene oxide dispersion A through ultrasonic dispersion. Then, ferrous sulfate heptahydrate was added into the dispersion A, stirred and dissolved to obtain a dispersion B of ferrous sulfate, graphene and water. Wherein the mass ratio of graphene oxide to ferrous sulfate is 0.02:1.
[0053] Then, according to the concentration ratio of urea and ferrous sulfate as 20:1, add urea into the dispersion B, stir to completely dissolve the urea, and obtain the suspension C.
[0054] Step 2: transfer the suspension C to a polytetrafluoroethylene-lined stainless steel reactor, and then place the reactor in a blast oven at 100° C. for 4 hours. The obtained reaction product was repeatedly washed with deionized water and ethanol to obtain a ferrous carbonate / graphene composite material, wherein the mass ratio of ferrous carbonate to graphene was 14.4:1.
[0055] Figure 7 The TEM image of...
PUM
| Property | Measurement | Unit |
|---|---|---|
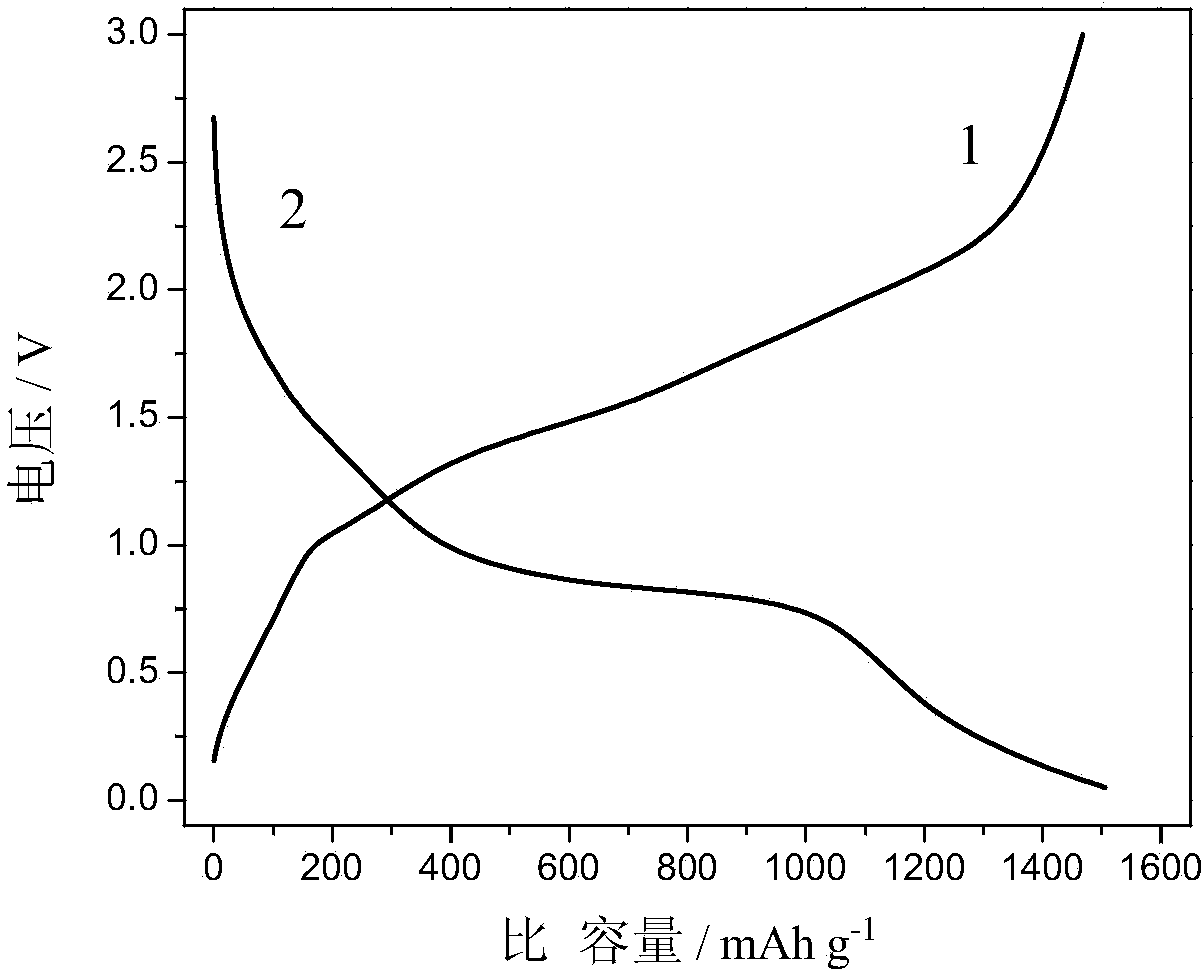
| First discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com