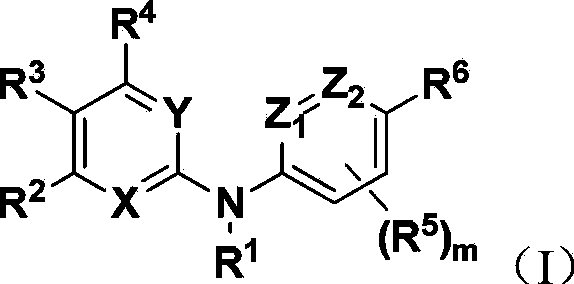
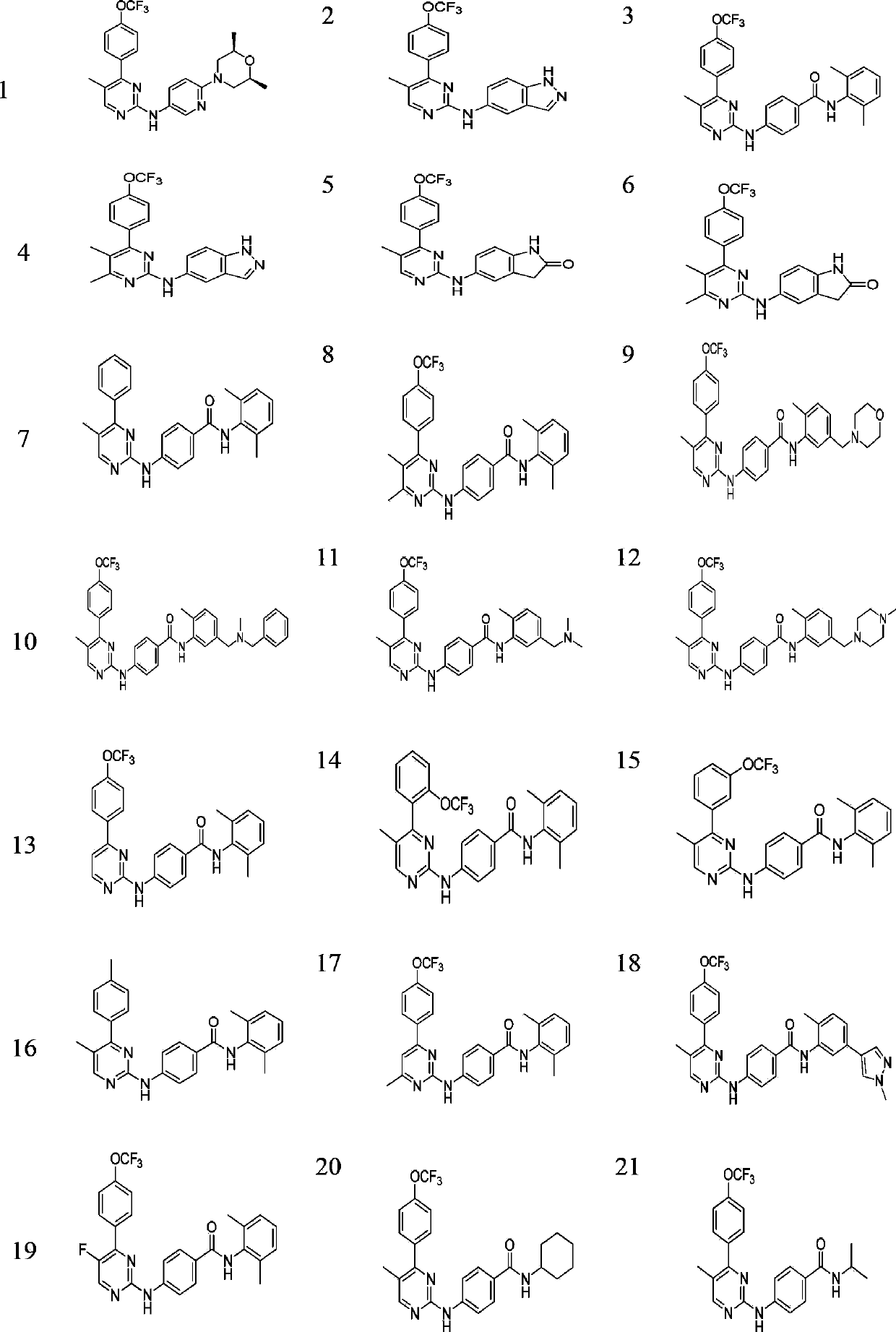
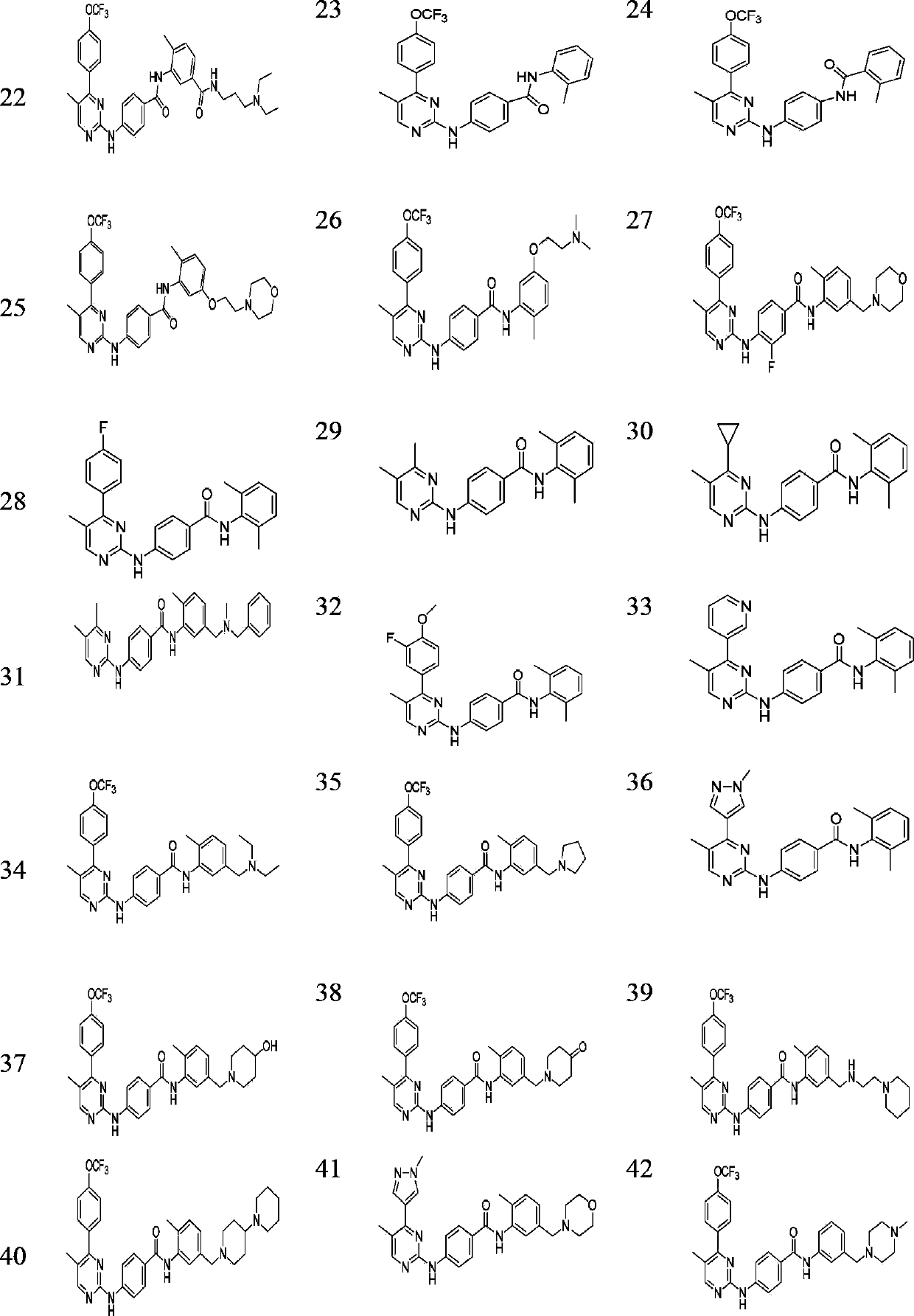
Pyrimidinamine and pyridinamine Hedgehog signal conduction inhibitors
A compound and alkyl technology, applied in the field of Hedgehog signaling pathway inhibitors and heterocyclic amine derivatives, can solve problems such as narrow indications, underdeveloped medical value of Hh inhibitors, and drug resistance problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0180] Preparation of compound 1
[0181]
[0182] Preparation of 3-nitro-6-((2S,6R)-2,6-dimethylmorpholinyl)pyridine (intermediate 1-b)
[0183] Weigh 2-chloro-5-nitropyridine (1-a, 1.58g, 1eq) and DMF (10mL) into a 50mL three-necked flask, add 2,6-dimethylmorpholine (1.15g, 1eq) , K 2 CO 3 (2.76g, 2eq), after the addition was completed, the temperature was raised to 80°C under the protection of nitrogen, and the reaction was refluxed for 12h. After cooling, ice water (50mL) was added and filtered to obtain a yellow solid (2.30g, 97%). MS(ESI)m / z:[M+H] + =238.0. 1 H-NMR (400M, DMSO-d 6 )δ8.97(d,1H,ArH),8.24(dd,1H,ArH),7.00(d,1H,ArH),4.45(m,2H,(CH) 2 O),3.37-3.62(m,2H,CH 2 ),2.62-2.68(m,2H,CH 2 ),1.18(s,3H,CH 3 ),1.16(s,3H,CH 3 ) ppm.
[0184] Preparation of 3-amino-6-((2S,6R)-2,6-dimethylmorpholinyl)pyridine (intermediate 1-c)
[0185] Weigh intermediate 1-b (2.1g, 1eq) and place it in a 50mL bottle, add methanol (20mL), add 5% Pd / C (0.2g) in a hydrogen atmosph...
Embodiment 2
[0191] Preparation of Compound 2
[0192]
[0193] Preparation of N-(5-methyl-4-(4-(trifluoromethoxy)phenyl)pyrimidin-2-yl)-1H-indazol-5-amine (2)
[0194] Compound 2 (58 mg, 43%) was prepared in a similar manner to compound 1. MS(ESI)m / z:[M+H] + =386.0. 1 H-NMR (400M, DMSO-d 6 )δ12.89(s,1H,indazole-NH),9.57(s,1H,NH),8.43(s,1H,ArH),8.26(s,1H,ArH),7.98(s,1H,ArH), 7.85(d,2H,ArH),7.59(m,1H,ArH),7.52(d,2H,ArH),7.43(m,1H,ArH),2.22(s,3H,ArCH 3 )ppm, HPLC: 93.98%.
Embodiment 3
[0196] Preparation of compound 3
[0197]
[0198] Preparation of methyl 4-((5-methyl-4-p-trifluoromethoxyphenyl)pyrimidine-2-amino)benzoate (intermediate 3-a)
[0199] Weigh intermediate 1-f (81mg, 1eq) and place it in a microwave tube, add isopropanol (2mL), add methyl p-aminobenzoate (60mg, 1.5eq) and 1 drop of hydrochloric acid, react in microwave at 170°C for 2h, Filtration afforded an off-white solid (110 mg, 97%). MS(ESI)m / z:[M+H] + =404.0.
[0200] Preparation of 4-((5-methyl-4-p-trifluoromethoxyphenyl)pyrimidine-2-amino)benzoic acid (intermediate 3-b)
[0201] Weigh 3-a (7mg, 1eq) and NaOH (18mg, 2.5eq) into a 10mL bottle, add MeOH (5mL), H 2 O (1 mL), reacted at 65°C for 1 h, poured the reaction solution into ice water, adjusted pH=7, a solid precipitated out, filtered to obtain a white solid (70 mg, 97%). MS(ESI)m / z:[M+H] + =390.0. 1 H-NMR (400M, DMSO-d 6 )δ12.53(s,1H,COOH),10.08(s,1H,NH),8.53(s,1H,ArH),7.92-7.85(m,6H,ArH),7.56(d,2H,ArH), 2.52(s,3H,ArCH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com